Winter wheat response to rock phosphate and the role of calcium in phosphate availability
Jed Eberly, Asa Hurd
Montana State University, Central Agricultural Research Center, Moccasin, MT
Introduction
Using rock phosphate (RP) to meet crop P needs is more sustainable and has a lower environmental impact compared to orthophosphate. While research in the use of RP has been ongoing for the past 100 years [1, 2] there are several challenges that have limited the efficacy of this approach. These factors include soil chemistry, precipitation, temperature, organic C levels, acid type, RP granule size, and presence of S solubilizing bacteria [3-6]. Studies of RP and S application also vary greatly in the optimal ratio of RP:S. Ratios of RP:S range from 2:1-5:1, with pH being one of the major factors in determining the optimum ratio [3, 6]. Research has shown that in greenhouse studies with soils at field capacity or in areas of high rainfall (>1000 mm) and acidic soils, RP and S may be nearly as effective as water soluble P [3, 7]. However, in areas receiving <600 mm, RP is considered ineffective as a P source because of its low solubility [8].
Previous work has shown that removal of calcium (Ca) with a cation exchange membrane can enhance RP acidulation and lead to higher concentrations of water-soluble P [9]. Amorphous silica can adsorb Ca at low ionic strengths [10, 11] and may thus function as a Ca sink. To date, few studies have evaluated amorphous silica as a Ca sink in soil.
Recent advances in rock micronizing and granulation technologies have enabled enhanced rates of RP solubilization in soils. This purpose of this study was to evaluate the yield response of dryland winter wheat grown with micronized blend of RP and S as the sole P source and the efficacy of amorphous silica to remove Ca for enhanced RP acidulation. This work also characterized two different silica sources to determine Ca binding capacity and rates of Ca removal in solution.
Methods
Field Studies
Dryland winter wheat field studies were established at the Montana State University Central Agricultural Research Center, Moccasin, MT, USA (47.059931N, -109.950159W). The soil type at this site is a Danvers-Judith clay loam, a complex of well-drained fine, smectitic, frigid Vertic Argiustolls. Average annual precipitation in this region is 390 mm.
Winter wheat was planted in 1.5m x 5m plots in a Randomized Complete Block (RCB) design with 6 replicates. Wheat was planted 05 November 2020 and 01 October 2021 and harvested 03 August 2021 and 29 August 2022. Soil sampling was performed post-harvest to determine soluble P levels in the soil in-furrow and between furrows. Soil cores (0-15 cm) were collected with a 2.54-cm soil probe. Treatments consisted of PhoSul, a micronized and granulated blend of RP, S, and amorphous silica (PhoSul, Sugar City, Idaho, USA), standard grower practice of 50 lb/ac 10:20:20:10 (N:P:K:S) starter fertilizer, and a control which received no P fertilizer. All plots were fertilized with 120 lbs/ac urea to meet crop nitrogen needs.
Rock phosphate acidulation flask experiments
0.5 g rock phosphate was added to several conical flasks along with 20 mL of 1M sulfuric acid. After 49 days the mixture was neutralized to pH 7 and diluted to 100 mL with Milli-Q water. Water soluble phosphate was quantified colorimetrically using 100 µL oxidizing reagent (1 mL of 5N sulfuric acid, 0.3 mL of 32.4 mM ammonium molybdate, 0.6 mL of 99.9 mM ascorbic acid, and 0.1 mL of 4.1 mM potassium antimony tartrate) and 100 µL sample. The sample was allowed to oxidize for 15 minutes and then measured at 882 nm.
Calcium binding experiments
Laboratory flask studies were established to evaluate calcium removal from an aqueous solution and to determine the concentrations of amorphous silica needed for complete Ca removal. Column chromatography grade silicon dioxide was obtained from Chem-Impex (Wood Dale, IL, USA) and ignimbrite (72% SiO2) was obtained from Montana Grow (Missoula, MT, USA). 1.0 g calcium chloride was added to a conical flask along with 20 mL of 1 M hydrochloric acid. Once the calcium chloride had dissolved, the solutions were neutralized and diluted to 100 mL with Milli-Q water. 10 mL of the diluted solution was then allocated to several flasks along with 40 mL of Milli-Q water and 4 mL of 6M sodium hydroxide. 0.72 g of silicon dioxide was added to half the flasks, and 1g of ignimbrite (72% silica) was added to the other half. At the given time points, 100 µL of 1% calconcarboxylic acid was added to the flask which was titrated with 0.025 M EDTA. Calcium concentrations were calculated based on 1:1 binding capacity per mol.
Results
Field Experiments
Field experiments showed a significant (p<0.05) winter wheat yield increase with PhoSul in 2021 but not in 2022 (Figure 1). Mean wheat yields in 2021 were 27% with PhoSul compared to the controls. In-furrow citrate soluble P concentrations were equivalent between PhoSul and conventional starter fertilizer (Figure 2). Soil calcium levels average 3,200 ppm in 2021 and 4,800 ppm in 2022 (Figure 3).
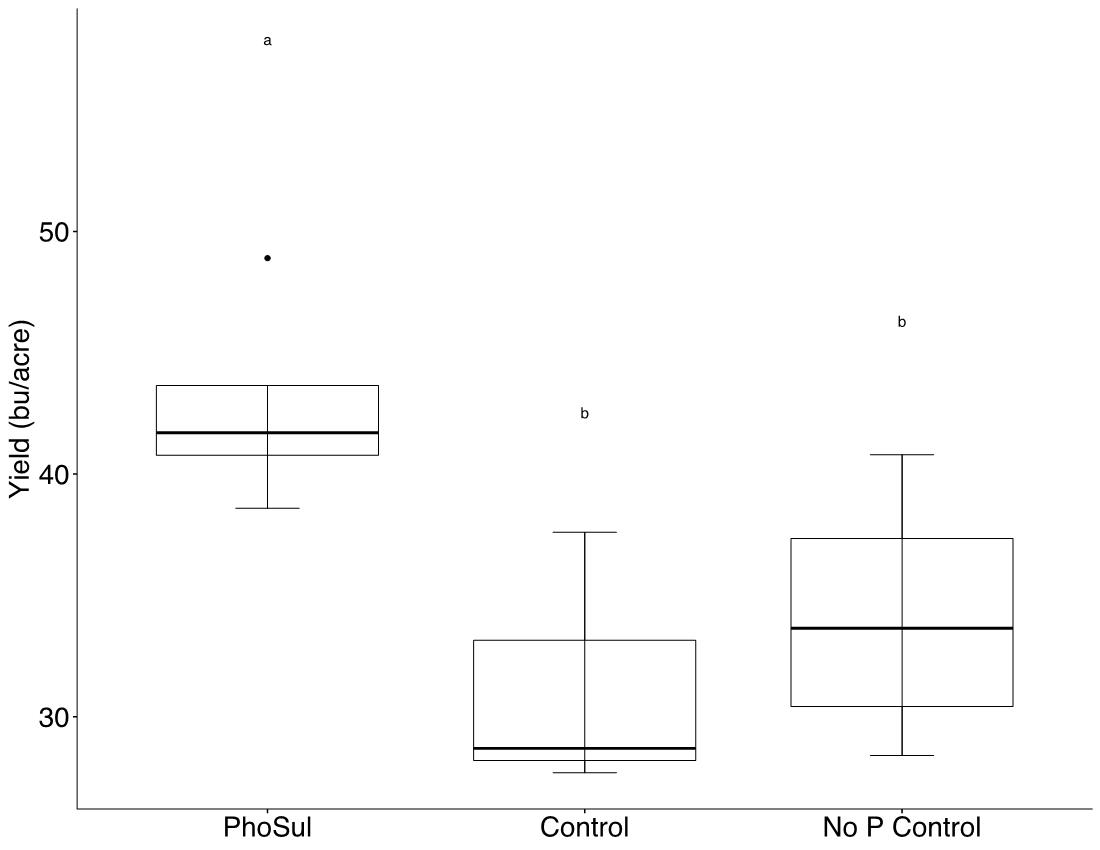
Figure 1A: Winter wheat yields in 2021.
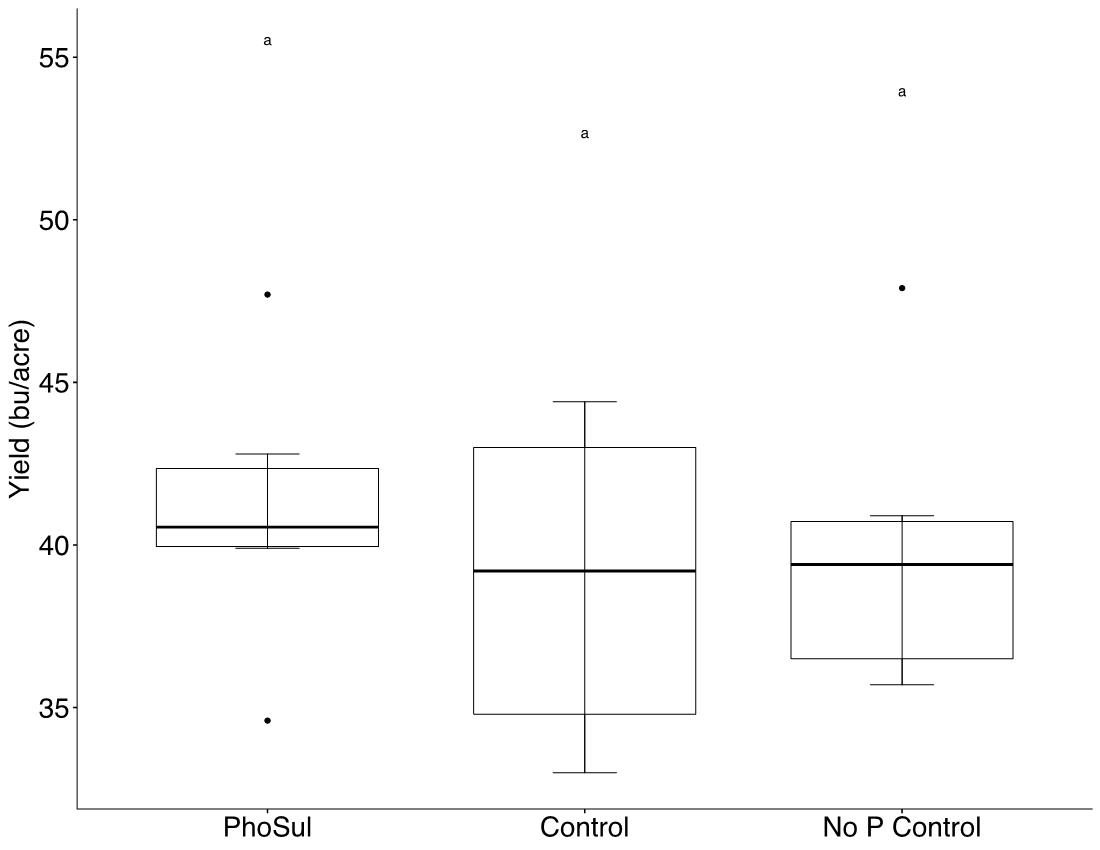
Figure 1B: Winter wheat yields in 2022.
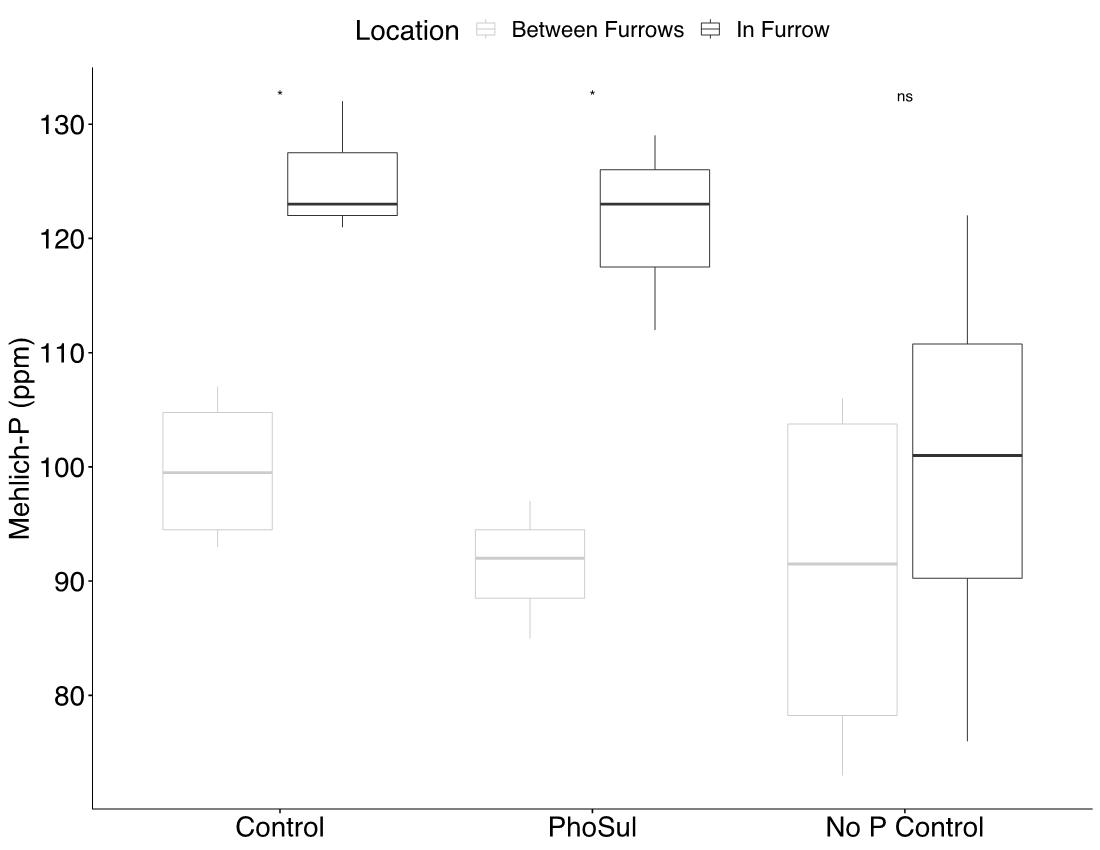
Figure 2: Mean citrate soluble P levels in-furrow and in bulk soil.
Laboratory Experiments
The results of the flask studies showed that both analytical grade SiO2 and ignimbrite were able to effectively remove Ca from solution. Ca was reduced below the level of detection within 6 hours with analytical grade SiO2 and within 72 hours with ignimbrite (Figure 4). RP acidulation studies showed that the addition of amorphous silica led to a significant (p<0.05) increase in water soluble P after 49 days (Figure 5).
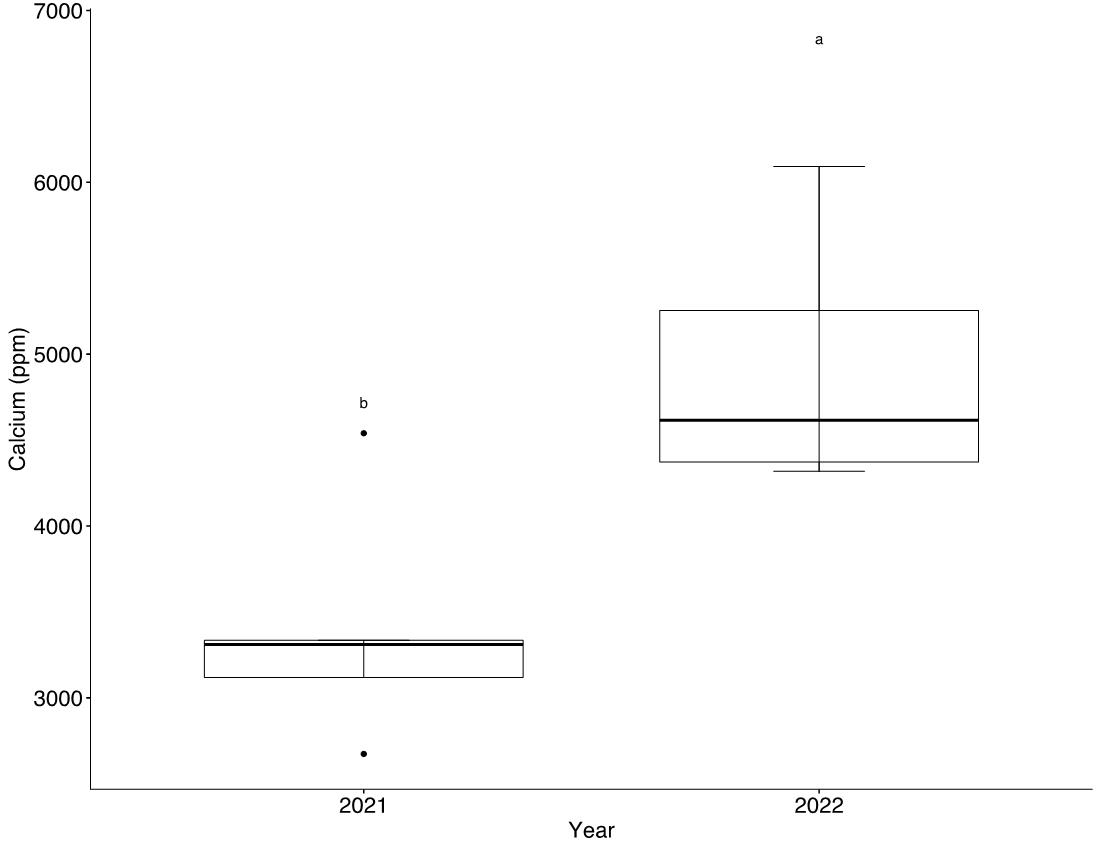
Figure 3: Soil calcium concentrations in 2021 and 2022.
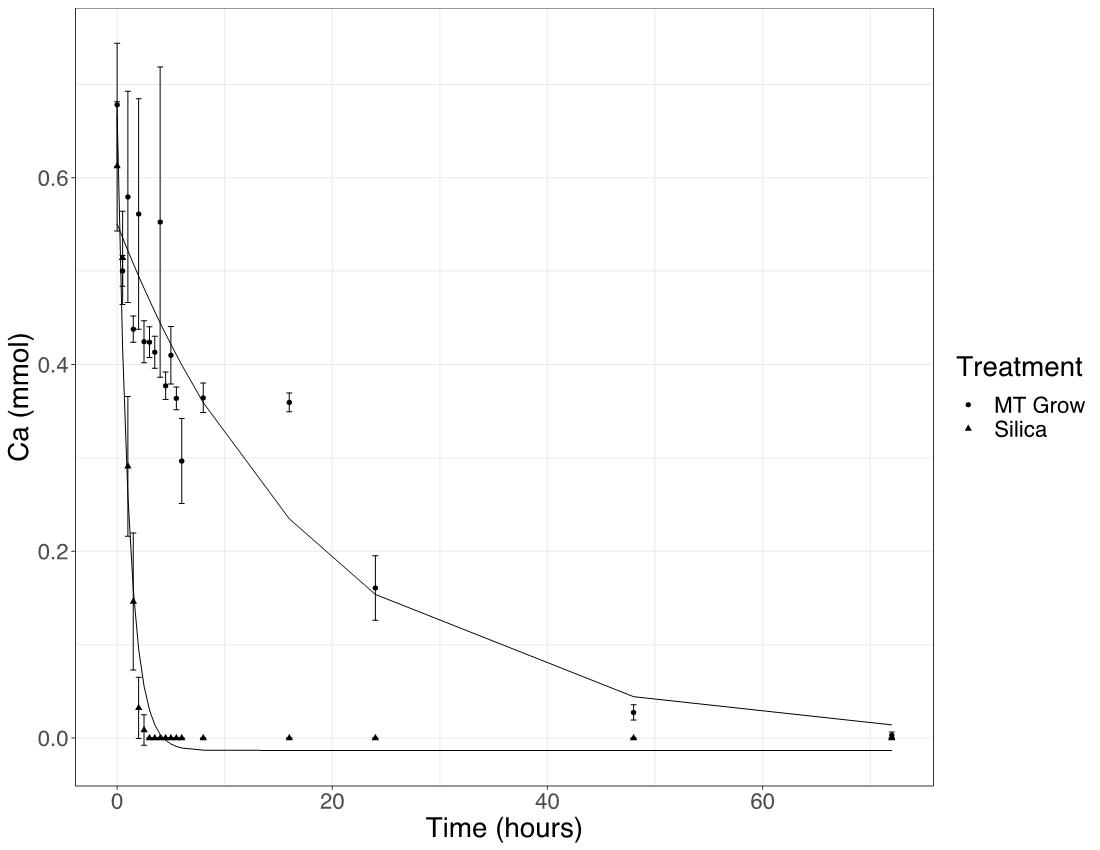
Figure 4: Calcium removal from an aqueous solution by analytical grade SiO2 and ignimbrite (MT Grow, 76% SIO2).
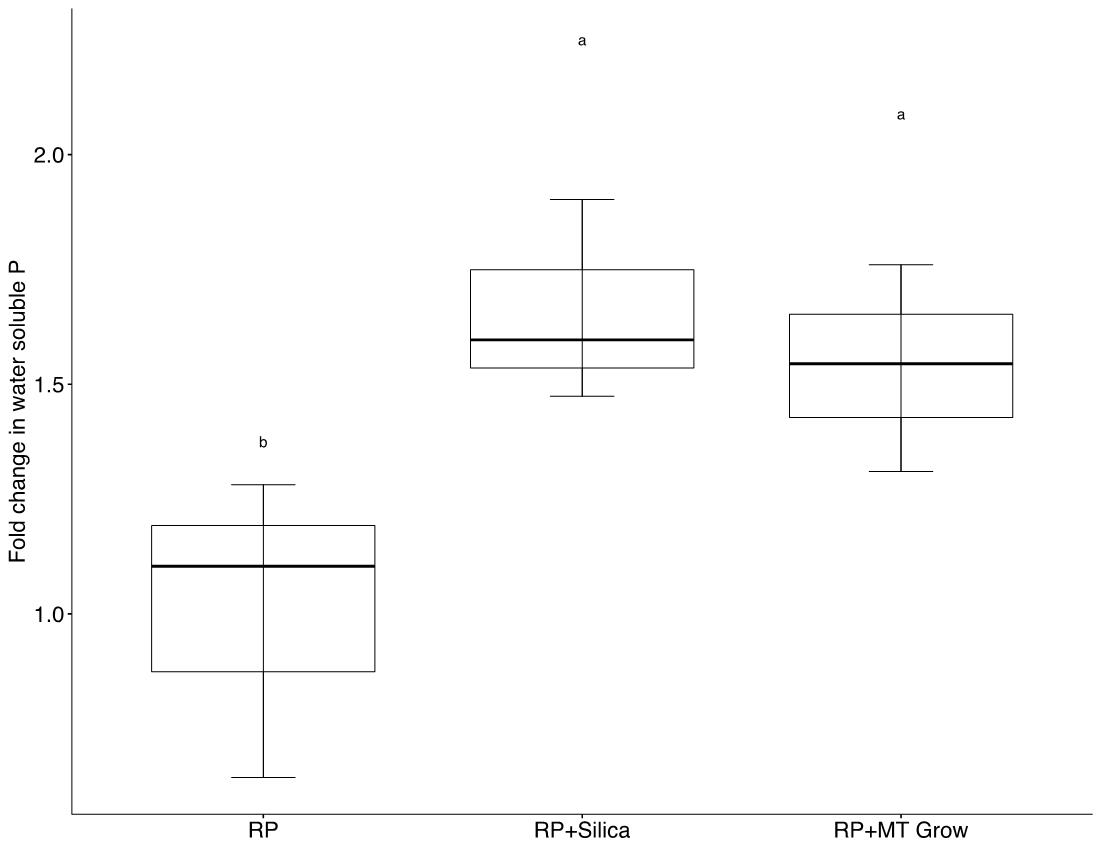
Figure 5: Fold change in water soluble P after 49 days. RP; micronized granulated blend of RP and S, RP+Silica; micronized granulated blend of RP and S with SiO2, RP+ Grow; micronizedMT granulated blend of RP and S with MT Grow (76% SiO2).
Conclusion
PhoSul, a micronized and granulated homogeneous rock phosphate product containing sulfur and amorphous silica was able maintain or enhance winter wheat yield relative to a conventional (MAP) fertilizer and an unfertilized control. Response to rock phosphate appeared to be dependent on soil Ca concentrations (Figure 3). Separate lab studies confirmed that amorphous silica contained in PhoSul can effectively bind Ca (Figure 4). Laboratory experiments also suggest calcium binding by silica may increase water soluble calcium released during rock phosphate acidulation (Figure 5)
Experiments are underway to verify the Ca response in the field and to determine the amount of amorphous silica needed to reduce Ca concentrations to allow complete acidulation of the rock phosphate in a variety of soils.
Acknowledgements
This work was supported by PhoSul LLC, 453 Business Loop, Sugar City, Idaho and the USDA hatch program, project number MONB00512.
References
- Lipman, J., G. and H. McLean, C., Vegetation experiments on the availability of treated phosphates. Soil Science, 1917. 2(6): p. 499-538.
- Lipman, J., G. and H. McLean, C., Experiments with sulfur-phosphate composts conducted under field conditions. Soil Science, 1918. 4: p. 243-250.
- Evans, J., L. McDonald, and A. Price, Application of reactive phosphate rock and sulphur fertilisers to enhance the availability of soil phosphate in organic farming. Nutrient Cycling in Agroecosystems, 2006. 75(1-3): p. 233-246
- Hammond, L.L., S.H. Chien, and J.R. Polo, Phosphorus availability from partial acidulation of two phosphate rocks. Fertilizer Research, 1980. 1: p. 37-49.
- Lewis, D.C., P.W.G. Sale, and D. Johnson, Agronomic efectivenes of a partialy acidulated reactive phosphate rock fertiliser. Australian Journal of Experimental Agriculture, 1997. 37: p. 985-993.
- Matamwa, W., C. Guppy, and G. Blair, In Situ Acidulation of Rock Phosphate. Communications in Soil Science and Plant Analysis, 2018. 49(4): p. 426-432.
- Chien, S.H. and L.L. Hammond, Agronomic effectiveness of partially acidulated phosphate rock as influenced by soil phosphorus-fixing capacity. Plant and Soil, 1989. 120: p. 159-164.
- Bolland, M.D.A. and R.J. Gilkes, Rock phosphates are not effective fertilizers in Western Australian soils: a review of one hundred years of research. Fertilizer Research, 1990. 22: p. 79-95.
- Robinson JS, Syers JK. A Critical Evaluation of the Factors Influencing the Dissolution of Gafsa Phosphate Rock. J Soil Sci (1990) 41:597-605.
- Pivovarov S. Adsorption of Ions onto Amorphous Silica: Ion Exchange Model. J Colloid Interface Sci (2008) 319(1):374-6. Epub 2007/12/11.
- Maraghechi H, Rajabipour F, Pantano CG

