Trapping click beetles with pheromone traps (Coleoptera: Elateridae)
Principal Investigator: Dr. Gadi V.P. Reddy Cooperators: Dr. Brian Thompson, Dr. Sindhu Krishnankutty
Western Triangle Agricultural Research Center, Montana State University, 9546 Old Shelby Rd.,
P.O. Box 656, Conrad, MT 59425, USA
Background:
Wireworms are the soil dwelling larval stage of click beetles (Coleoptera: Elateridae). In this larval stage click beetles are a significant pest of a wide variety of crops grown in Montana. Wireworms have seen a recent reemergence due in large part to the loss of Lindane, a primary chemical control agent, due to toxicity concerns. The loss of Lindane and the reemergence of wireworms as a pest necessitates new ways of evaluating pest presence and control measures.
Pheromones are chemicals released by insects to communicate between members of a particular species. These highly specific compounds elicit attraction between sexes in click beetles and can be used to monitor population occurrence and density in agricultural fields. For the click beetles of the Northern Great Plains, pheromone based monitoring has not been developed. Development of this technology is crucial to monitoring and control programs. This ongoing research looks at the development of pheromone based trapping systems for Limonius californicus and Hypnoidus bicolor, two dominant wireworm species found in Montana.
Although no detailed work on the use of pheromone compounds has been done in the USA, there have been several reports available on related wireworms species found in Europe. Pheromone compounds appear to be similar in its related insect species. In this context, we attempted to lure in click beetles using chemical lures used to trap click beetles in Europe. In 2014, the work built on trapping data from the previous flight season of 2013 and will be continued again in the spring of 2015 ahead of the grant expiration deadline.
In 2013 sex pheromones from European Agriotes species of click beetle were tested for their ability to attract Limonius and Hypnoidus species in wheat fields around Conrad, MT. These studies showed that the local species exhibited no cross attraction with these sex pheromones. In 2014 we attempted plant volatile based attractants.
Methods:
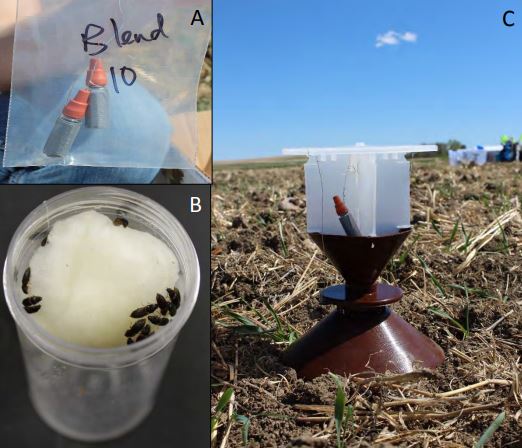
In 2014, we set out traps in a circular array and baited them with plant oil extracts shown to have attractive properties in Agriotes species. Cis-3-hexene-1-ol, (Z)-3-Hexenyl acetate, Methyl benzoate, Methyl salicylate were tested alone and in combinations along with a control by filling 5 dram vials with a 0.5 Molar concentration of these plant oil extracts and capped them off with rubber septa. Odor release rates are highly dependent upon temperature and were not calculated, though a detectable smell was noticeable for each vial. Vials were wrapped in duct tape to keep UV light out and were individually wrapped in foil and placed in -20˚F freezer until needed (Figure 1A). There were three replicates of each volatile (n=30 trap total). Each trial consisted of the attractants randomly ordered on a circular array with the attractants hung from the exterior of the Yatlor trap (Figure 1C). Each trap was a minimum of 10m from the next trap. Each of the three arrays was a minimum of 25m from the next array. Traps were checked every two days for the presence of click beetles.
In addition to volatile traps pitfall traps were placed in an array of 10 traps placed 10 meters apart in a square grid pattern. Pitfall traps were deployed 30m from Yatlor traps in the same field and were checked every two days. Click beetles taken from pitfall traps (Figure 2A) were used to bait cages on Yatlor traps (Figure 2B). Beetles were held in captivity on a diet of honey water (1:10) soaked cotton balls (Figure 1B). Two types of pitfall traps were tested, bucket traps and funnel traps.
The location of traps in one large wheat field was determined by spring scouting for wireworms and a historical presence in that field. The land was owned by the Devries family and plots were approximately located at GPS coordinates 48.182867, -111.805412.
Results:
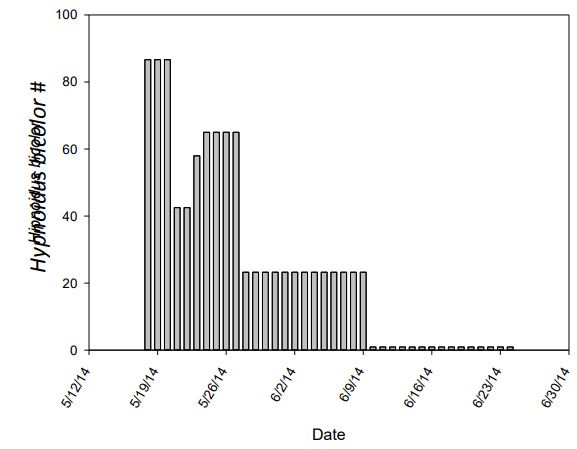
Catch data was highly skewed by catch results. The only traps to catch any click beetles were the bucket pitfall traps. These traps were unbaited by any scents or plant material. Bucket traps caught 979 Hypnoidus from May17th - June 23rd (Figure 3). Hypnoidus beetles placed in cages did not attract more beetles, neither did the plant compounds. The effectiveness of the Yatlor traps is questionable. This trap was proved to be effective in catching the click beetles in Europe. No statistics were performed on the data as the only trap to catch beetles was the pitfall trap.
Discussion:
Pitfall traps are passive traps that catch beetles that happen to fall into them. The beetles caught in the pitfall traps signify a large population of pestiferous species in the field, however the plant volatiles failed to catch any beetles. This failure may be due to the inadequacy of the Yatlor trap or of the plant volatiles. Pitfall traps will be used in combination with pheromones and plant volatiles in the coming spring in an attempt to test attractants with an effective trap. Adults collected in the spring of 2015 will be dissected and pheromone glands will be sent for analysis of chemical composition. These compounds will then be synthesized and analyzed in the following spring. The Western Triangle Agricultural Research Center (WTARC) will test the attractiveness of volatile chemicals such as pheromones in a more controlled environment using an olfactometer (Figure 4) that is currently being installed at the Center. Olfactometer confront insects in a special chamber in a controlled fashion where exact responses either positive or negative can be recorded and should eliminate much of the variability that currently confounds pheromone trapping of Hypnoidus and Limonius click beetles in this area.
WTARC is also in the process of understanding pheromone composition of local species of click beetles. This will eventually help us to develop synthetic pheromones that will be used in pest monitoring and mass trapping. Accurate identification of species is crucial as pheromones are species-specific. Poorly known click beetle taxonomy, variability in the presence of different species of beetles based on geography, crop, and soil type makes it difficult to identify the species accurately. WTARC has set up a DNA work room in 2014 (Figure 5). New genetic methods will be used as an efficient taxonomic tool to evaluate variability in species and subspecies in the area.
Acknowledgments:
This work was supported by Montana Wheat and Barley Committee and U.S. Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA) Hatch (#MONB00859).
Figure 1. A) Plant volatile release vials, B) Click beetles feeding on honey water (1:10) soaked cotton ball in storage vial, C) Yatlor trap deployed in spring wheat field.
Figure 2. A) Pitfall trap, B) Yatlor trap with cage affixed to top to hold live insects.
Figure 3. The raw number of Hypnoidus bicolor click beetles caught in bucket pitfall traps daily.
Figure 4. Olfactometer with decision chamber and collection ports installed. Air leaves through the hole in the center and is pumped in through the collection ports. The test insect follows attractants to their source and is collected in the glass collection chamber.
Figure 5. DNA work room at WTARC: A) PCR station; B) Gel electorphoresis area