Comparative evaluations of three entomopathogenic nematodes against Tenebrio molitor (Coleoptera: Tenebrionidae), for use in management of wireworms (Coleoptera: Elateridae) in Montana wheat fields
Principle Investigator: Dr. Gadi VP Reddy Cooperators: Dr. Scott L. Portman* and Amber Ferda
Montanan State University, Western Triangle Agricultural Research Center, Conrad MT, 59425
Introduction
Wireworms, the larval stage of click beetles (Coleoptera: Elateridae), live in the soil and attack the roots of germinating wheat seedlings during the spring months. The damage caused by these insects is typically variable but can be as severe as 28% loss in spring wheat yields. Historically, wireworms have been difficult to control because these insects can burrow deep into the soil column in response environmental factors such as temperature and moisture. One possible control method for wireworms might be entomopathogenic nematodes (EPNs). These microscopic roundworms have the ability to move through the soil as they search for insect prey. However, EPNs are not well known to infect and kill wireworms because of the insect’s hardened cuticle and well protected entry points such as spiracles, mouth or anus. During the winter months, wireworms are difficult to collect in the field because they burrow deep into the soil to avoid the freezing temperatures in Montana. Nevertheless, some species of Tenebrionidae, such as Tenebrio molitor (mealworm), have similar morphology to wireworms. Mealworms could serve as a good substitute for wireworms to test the ability of different EPNs to infect insects with hardened cuticle and well protected entry points.
Objectives:
-
Test three species of EPNs for their ability to infect and kill Tenebrio molitor (mealworm) larva and pupae, as a proxy for wireworms.
Materials and methods
Tenebrio molitor larvae and adults were obtained from Carolina Biological Supply Company (Burlington, NC). Larvae were separated into early and late instars and transferred to 33.7 cm × 21.8 cm × 11.9 cm (L×W×H) plastic containers and reared on a diet of oat bran. Adults were housed in a 475 ml plastic deli cup with 2.5 cm of oat bran on the bottom for egg laying. Potato slices were provided in each container as a source of moisture. Mealworm colonies were stored in a 23oc incubator prior to treatment.
Just before treatment, 30 late instar larvae, 30 early instar larvae, and 30 pupae were removed from the mealworm colonies. For each EPN being tested, ten individuals from each development stage were transferred to the bottom of three clean 475 ml deli cups. Due to an insufficient number of available pupae, S. feltiae was not tested with Tenebrio pupae (Table 1). A thin layer of oat bran was added to the bottom of the deli cups housing larvae to provide them with a food source. Approximately 5 cm of autoclaved soil was added on top of the insects.
Table-1: Experimental design of the entomopathogenic nematode treatments with Tenebrio molitor.
Three species of commercially available EPNs (Steinernema feltiae, S. riobrave, and Heterorhabditis bacteriophora) were ordered from suppliers and stored at 8oC. EPNs were allowed to equilibrate to room temperature (22oC) before being added to 3ml of distilled water. EPN solutions were first mixed thoroughly and sucked up into a disposable pipette. EPNs were added to the deli cups containing larvae and pupae by applying 10 drops of the solution, randomly, to the surface of the soil. After EPN application, deli cups were incubated at 20oC in a growth chamber for 5-14 days. After 5 days, dead or dying larvae and pupae were removed from the deli cups and placed individually in small 75 ml plastic portion cups for several days (2-4 days) of observation. After the observation period, dead larvae and pupae were assayed for nematode infection.
Results and discussion
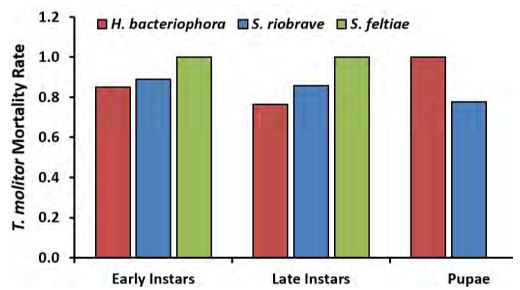
The experiment was replicated two times. For each replication, mortality rates for all treatment combinations (insect development stages × nematodes) were calculated. Average mortality rates were calculated from the two replications (Fig. 1). On average, mortality rates were above 75% for all EPN treatments. Larvae (late and early) treated with S. feltiae exhibited the highest mortality at 100%. Interestingly pupae treated with H. bacteriophora, and S. riobrave also showed high mortality (100% and 77.8% respectively). Due the similarity in morphology between mealworms and wireworms, these results suggest that wireworms may be susceptible to infection by the three EPN species tested here.
Because only 2 independent samples were obtained for each treatment, no statistical tests could be performed on the data. More replications will be conducted in order to obtain enough data for more robust statistical analysis. In this study, the larvae were free to move through the soil at libitum, presumably coming in close proximity to the nematodes at the surface. To better understand how EPNs migrate through the soil, future work could test their ability to infect insects at different soil depths. Future work (spring 2016) also includes collecting wireworm samples from wheat fields in NW Montana and testing the infectivity of the three EPN species discussed in this report.
Figure 1 Bars represent average mortality rates of different development stages of mealworms (Tenebrio molitor), treated with three species of entomopathogenic nematodes.
Acknowledgements
This work was supported by Professional Development Program (PDP) of the USDA-Western SARE project #2014-38640-22175/Utah State University sub award# 140867038 and USDA- National Institute of Food and Agriculture Hatch (Accession#MONB00859) for funding. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA).