Determining the prevalence of the entomopathogenic fungus, Beauveria spp., in diapausing wheat stem sawfly larvae
Principle Investigators: Dr. Gadi V.P. Reddy1 and Dr. Stefan T. Jaronski2 Project personnel: Amber Ferda1 William Franck2 Rob Schlothauer2
1Western Triangle Agricultural Research Center, Montana State University, 9546 Old Shelby Rd., P.O. Box 656, Conrad, MT 59425, USA
2United States Department of Agriculture, Agricultural Research Service, Northern Plains Agricultural Research Laboratory, 1500 N. Central Avenue, Sidney, MT 59270
Introduction
In 2013 Jaronski and Reddy discovered a number of WSS larvae with infections by the insect pathogenic fungus, Beauveria infection in the course of some laboratory observations. This is the first record of Beauveria infecting WSS in North America; there is an obscure report from Romania about Beauveria from Cephus pygmaeus.
The resulting 8 isolates were genotyped by USDA ARS in Sidney MT, and identified as being in three clades of B. pseudobassiana. In addition, three isolates were made of Metarhizium, subsequently identified by ARS as M. pemphigi. Subsequently, a limited survey was conducted in two fields in Fall of 2013 and processed by USDA in Sidney. From this survey 22 of 25 live larvae extracted from their hibernacula in one sample and 5 of 5 larvae from the second sample were positive for Beauveria (Fusarium spp. on the other hand, was rare, 1/25 in the first, 0 in the second USDA fall sample. These isolates have also been isolated and genotyped by USDA; these isolates fall into several clades of B. bassiana as well as B. pseudobassiana.
This report parallels and overlaps that of Dr. Jaronski for his grant from the wheat and barley Committee. This ongoing study is funded through spring 2015, although the parallel grant to USDA ARS in Sidney extends to December 31, 2015, and has been extended through 2015.
Methods
Fields were sampled in the Spring and Fall of 2014. In Spring 2015 the fields where the Beauveria was originally recovered were sampled. In each field approximately 100 stubs (wheat stem sawfly hibernacula) were collected along a transect parallel to the field edge. These stubs were shipped to ARS Sidney MT for processing. There the larvae were extracted. Dead and live larvae were decontaminated by immersion in 0.5% NaOCl, followed by two rinses in sterile water after which the excess liquid was blotted off and the larvae placed on water agar for incubation at 25° C. Any insects developing white fungal outgrowth, esp. those that had previously turned a pink color, characteristic of Beauveria infection, were isolated for further fungal outgrowth and sporulation.
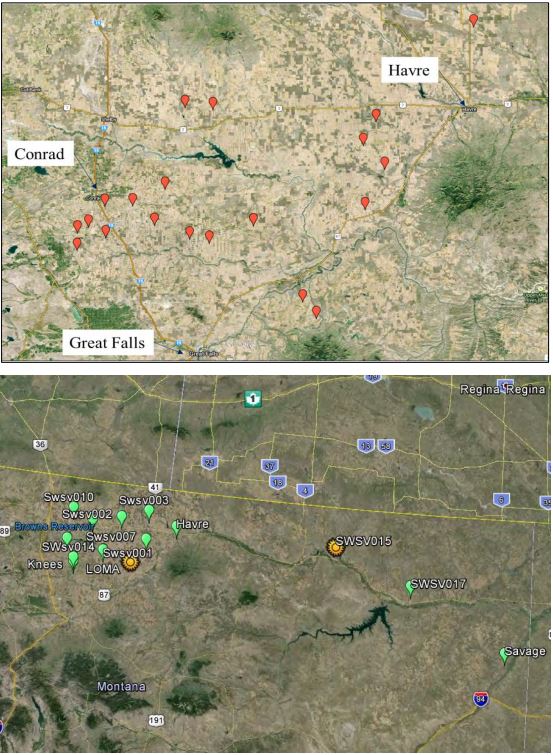
In the fall of 2014 the WTARC research team collected wheat stubbles from 20 different locations across five counties in north central Montana (Figure 1). In addition, USDA made parallel collections in 15 fields in eastern Montana. Every effort was made to collect at least 100 stubs from each field along transects to accommodate potential heterogeneous distribution of the fungus. In some heavily infested fields this was easy. In others, where sawfly populations were lower, considerable effort was needed to collect the desired number.
Additional collections were made in the Spring and Fall of 2015, in or adjacent to fields from which we had isolated Beauveria in 2013 and 2014. Stubs collected by WTARC were then sent to the USDA insect pathology lab of Dr. Stefan Jaronski, the Co-PI, in Sidney, MT. WSS larvae were carefully dissected from their hibernacula, surface decontaminated (1 minute immersion in 0.5% NaOCl followed by two rinses in sterile water), and incubated on 20% water agar for 7-10 days. Any larvae turning pink (a diagnostic sign for Beauveria infection) or otherwise dying were transferred to individual plates to allow any Beauveria, if present, to emerge and sporulate. Larvae were then placed on selective media for fungal isolation, then transferred to separate culture dishes and catalogued for identification and long-term storage. Molecular typing for assignment of isolates to Beauveria species and subspecies clade, as well as evaluation of infectivity/mortality followed initial isolation. Approximately 3,500 sawfly larvae were processed by ARS in this survey.
Seventeen new Beauveria isolates were obtained from the Fall 2014 samples. Another 41 isolates were made in 2015 from the original (2013, 2014 positive) fields. Few Fusarium spp. infections were seen. Overall prevalence of these fungi was very low — 2 of 35 fields sampled — but when present the prevalence was very high. Unexpectedly, infections by the entomopathogenic fungi were not immediately evident in living larvae, but only after one or two days. All fungi infecting larvae were isolated, cultured and accessioned into the USDA ARS Entomopathogenic Fungus Collection (Ithaca NY) as well as at USDA NPARL, Sidney.
Identification of all these isolates by molecular means (B-locus gene sequence) classified them as Beauveria pseudobassiana and B. bassiana. Beauveria pseudobassiana was by far the more common. All the isolates fall into three closely related but distinct groups. Apparently, the Beauveria isolates from each field are more closely related to each other than to isolates from the other fields, being, in a sense, island populations. Further identification and taxonomic differentiation by ARS, using high resolution Arbitrary Fragment Length Polymorphism, is not yet complete.
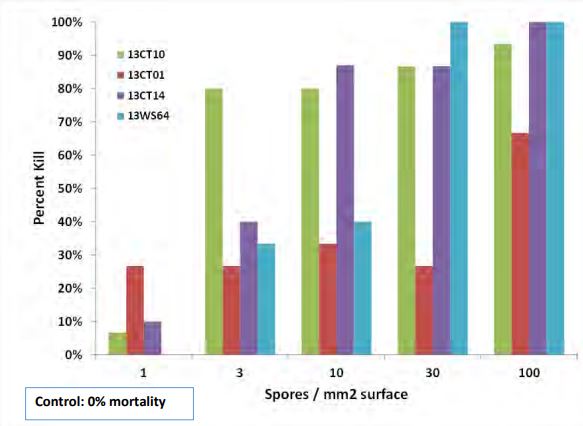
Replicated larval wheat stem sawfly bioassays were conducted by ARS using three isolates of B. pseudobassiana and one of M. pemphigi (isolated in 2013). In brief, exposure of last instar larvae from a disease-free field consisted of having healthy larvae crawl over filter paper impregnated with spores of the fungi, at densities of 1, 3, 10, 30, 100 spores /mm2 of surface.
Larvae were subsequently incubated for 5-7 days. Any dying of fungus infection were isolated and incubated at high humidity and room temperature for 5 days to elicit emergence of the fungi for identification and isolation. Conveniently, larvae infected by Beauveria turn a distinct red color just before death, followed by characteristic fungal outgrowth on cadavers and spore production. In all cases the infecting fungi covered their victim’s bodies with characteristic mycelial growth and spores. All the fungi were very infectious and pathogenic for the WSS larvae, with two isolates causing 80% larval mortality at 3-10 conidia/mm2 surface area and remaining isolates highly so at 30-100 conidia/mm2.
Results
Our survey has indicated that the occurrence of Beauveria in wheat stem sawfly larvae is rare. From the beginning of this research, Beauveria presence in larvae has been identified in only 4 fields. While DNA studies by ARS are not complete, it may be that the origin of the Beauveria is from the soil in the affected fields. ARS isolated Beauveria from the soil attached to collected stubble and is currently determining their taxonomic identity. In retrospect, our sampling procedure, generally targeting fields with high levels of sawfly infestation with the goal of efficient collection, may have biased the observations. The Beauveria was found in fields with few larvae, and when present had a very high prevalence. It is possible that Beauveria has hitherto been an invisible mortality factor repressing sawfly populations in specific fields.
The bioassays conducted by ARS indicate considerable pathogenicity of the tested fungi. An implication of the bioassay data is that the Beauveria, if indeed endophytic in wheat as implied by ARS from their other MWBC-grant-funded experimentation, would not have to greatly grown out and sporulated in the interior of a wheat stem to infect a copresent sawfly larva. Larval mortalities from exposure to 1-10 conidia/mm2 of surface area, esp. within 5 days of exposure, are exceedingly low in the experience of the ARS Co-PI.
ARS is bioassaying additional Beauveria isolates as supplies of diapausing sawfly larvae become available and as the additional fungus strains are identified as unique from the others. ARS is also subjecting the Beauveria isolated from stubble in Beauveria-positive and negative fields to determine if there is a relationship between observed fungus prevalence and genetic identity of the fungus strains.
Acknowledgments:
This work was supported by Montana Wheat and Barley Committee. Steve Rehner, USDA ARS Beltsville MD verified the taxonomic identification and B-locus-based clade structure of the Beauveria.
Figure 1. 2014-15 Montana collection sites. (top) Collections by WTARC; (bottom) collections made by ARS
Figure 2. (left) Sawfly larvae infected by Beauveria showing the characteristic coloration of infection; (center) emergence of Beauveria from cadavers with continued incubation at high humidity; (right) sporulation of Beauveria upon maturation of the fungus on cadavers.
Figure 3. In vitro efficacy of three Beauveria pseudobassiana (13CT01, 10, 14) and one Metarhizium pemphigi (13WS64) for sawfly larvae. Data are mean larval mortalities 5 days after larval exposure to filter paper impregnated with 1, 3, 10, 30, or 100 conidia/mm2 surface area.