Effect of temperature on two bio-insecticides for the control of confused flour beetle (Coleoptera: Tenebrionidae)
Principal Investigator: Dr. Gadi V. P. Reddy.
Cooperators: Dr. Brian M. Thompson, Deb Miller and Daniel E. Picard
Western Triangle Agricultural Research Center,
Montana State University,
9546 Old Shelby Rd,
P.O. Box 656,
Conrad, MT 59425
Introduction
Globally, 10-20% of all grain produced is lost to stored product pests before it reaches the consumer. Climate plays an important role in grain storage as it interacts with the grain and the pests that consume it. Bacteria, fungi, and insects, the primary pests of stored grain, are highly responsive to changes in climate. Climate affects the growth rate, reproduction, mortality and geography of pests. The interconnectedness of climate and biology has predicted changes in distribution, phenology, and abundance of many animals, and stored grain pests are likewise expected to adapt to their changing surroundings. Changes in climate may also alter current and future control strategies for stored grain pests as changes in temperature, rainfall and crops all adjust to new climate paradigms.
Figure-1: Interaction between temperature and bio-based insecticides on Tribolium confusum
At high latitudes, seasonally cold ambient air temperatures are conducive to physical control of grain pests through desiccation and freezing. Cold air is continuously pumped through the grain mass in storage using fans. A continuous supply of cold dry air desiccates and freezes flour beetle larvae and adults of the confused flour beetle, Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae). Heated or cooled air treatments allow more rapid alterations of the internal environment of the grain bin, which is usually well buffered from external temperature. Physical control using heated or cooled air is only applied when outside air temperature provides a natural source of conditioned air. Under a warming climate, forced air circulation may lose efficacy, especially at higher latitudes and higher elevations where winter temperatures are expected to moderate over the coming century.
The development of new pesticides that reduce the risk of non-target effects (e.g., development of resistance, residue problems, etc.) are badly needed in grain production. Aluminum phosphide is currently the best control measure for almost all stored product species, but is highly toxic to humans and animals, and resistance has developed within T. confusum populations. Alternative control strategies for the control of stores grain pests are needed. Biologically based insecticides are an alternative that is gaining traction for their adaptability and safety compared with aluminum phosphide, but the effectiveness of biologically based control measures is reliant on their ability to function under various climate regimes.
Biologically based insecticides are an attractive alternative to physical control and aluminum phosphide insecticide. Spinosad is a bioinsecticide produced from the bacterium Saccharopolyspora spinosa Mertz & Yao that is increasingly used against stored grain pests. It consists of metabolites that are toxic via contact or ingestion. Once inside the insect body, spinosad excites the nervous system, causing paralysis and eventually death. Spinosad ingested with the stored grain is 5–10 times more active than spinosad encountered through surface contact.
Entomopathogenic fungi such as Beauveria bassiana, in contrast, work primarily on surface contact. Spores of B. bassiana attach to the insect cuticle when the insect brushes against the spores. Binding of active sites on the infective spore with the insect cuticle initiates germination and the start of the infection process. Entomopathogenic fungi may germinate in grain storage conditions through favorable microclimates on the insect’s body, where moisture levels are higher, to grow and infect. Oral, anal and respiratory orifices are moist microhabitats where fungal pathogens may enter the insect body and initiate infection. Though B. bassiana often displays host specialization, it can also be a generalist pathogen capable of infecting many insect species. Beauveria bassiana is reported to be effective for managing both Tribolium castaneum
T. confusum (Coleoptera: Tenebrionidae).
The differing nature of these biologically based insecticides for controlling insects at differing temperatures has not been examined. Changes in temperature can affect the reproduction, development and behavior of insects and their pathogens. Growth and reproduction of many stored grain pests is optimal at 25-33˚C. We tested the effectiveness of B. bassiana and spinosad against T. confusum, which is one of major stored grain pests in the Golden Triangle grain- growing region of Montana, USA, when exposed to varying temperatures.
Materials and methods
Source and rearing of insects
Adults of T. confusum were purchased from Carolina Biological Supply Company, Burlington, NC (USA) and reared on a 1:1 mixture of whole-wheat Durham grain and flour grown and processed at the Western Triangle Agricultural Research Center. Laboratory cultures were maintained for one month prior to use in experimental trials at which time all life-stages were present within the colony. Rearing containers were clear plastic storage containers (30 cm long × 9 cm wide × 5 cm depth) with < 1 mm diameter aeration holes in the lid. Colonies were held using environmental chambers (Shel Lab, Cornelius, OR, USA) at 20˚C and 50% RH under complete darkness until needed
Test arena
Tests were conducted in chambers modified from 60 mL sample vials (SecurTainer IITM, Simport Scientific, QC, Canada). Container lid centers were removed and lids were screwed down over the top of sterilized vellum cloth for ventilation. Between tests, all test vials and covers were sterilized with 70% ethanol and UV light for 24 h prior to use. Each vial received 1g of equal parts wheat flour and whole-wheat seeds identical to the rearing mixture. The test arena volume precluded microclimate variance, and wheat moisture was not measured during the experiment.
Larval response to temperature and insecticidal activity
Because the larvae are the most damaging life stage of T. confusum, this stage was chosen for all trials. Confused flour beetle in the last instar (9th) were selected from the rearing colony for use in the experiment. The larval age is determined based on the descriptions given in Park (1934). Ninth instar larvae are brown in color and 6.0 mm long, 0.69 mm broad across the head, and weigh 2.4 mg. Late instar larvae are very active feeders (Park 1934). We tested two biobased pesticide treatments: spinosad 80% (Entrust® WP; Dow Agro Sciences Indianapolis, IN, USA) and B. bassiana (BotaniGard® 22WP; Laverlam International, Butte, MT, USA) at 8, 16, 22, and 25 ˚C. Each temperature used in this study is typical of temperatures experienced in the local grain production area (central Montana) during grain harvest and storage. Temperatures outside this range are known to be detrimental to T. confusum survival. Each vial with 5 larvae (treatment × temperature combination) was replicated 5 times (for a total of 25 test larvae per pesticide × temperature combination). Biologically based insecticides were mixed with wheat grain and flour prior to adding test larvae. Beauveria bassiana was added at the label rate of 2×1013 spores per 0.45 kg of grain as dry material, so as not to alter moisture levels in grain.
Spinosad was added at the label rate of one part per million (ppm) dry powder. Test vials were held in the dark at one of the four experimental temperatures and at constant relative humidity of 52% in a Panasonic MLR-352H-PE plant growth chamber. Control insects were held under identical experimental conditions minus the addition of the biobased insecticides. Larvae were extracted from the wheat-flour mixture once a week to determine mortality or survival. Trials were continued until the larvae were either all dead or pupated (12-45 d depending on temperature treatment).
Response of adults of T. confusum to B. bassiana
Adult flour beetles were held at one temperature (22˚C) in the presence of B. bassiana under exposed conditions and in grain. Both assays took place in containers identical to those used for larvae. The grain mixture and B. bassiana concentrations were also the same as in the larval experiment. “Exposed” assays consisted of placing adult beetles on sterile filter paper in SecurTainersTM without grain. Beauveria bassiana treatments received 2×1013 spores per 0.45 kg of grain or, for the exposed containers that had no grain, this same quantity of spores but in vials without grain. The spores were counted under the microscope. Beetles were held in the dark and monitored for mortality. Mortality was assessed by gently squeezing the beetles with forceps to look for movement. After 28 d, mortality was assessed for all treatment times. Ten beetles were tested in each replicate container. There were five containers for each treatment. Dead beetles were placed in petri dishes with sterile filter paper and 100 µl of sterile water, and fungal growth was monitored for the presence of B. bassiana.
Statistical analysis
The percentage mortality was calculated for each replicate as the number dead out of the original five placed in each replicate container. Percentages were arcsine transformed before statistical analysis to correct for the assumption of normality and percentage data. Analysis of covariance was used to determine whether or not treatments affected survival across the time period using the aov command in R for the model at each temperature. Post-hoc analysis was conducted with Tukey’s HSD test for multiple comparisons. Statistical significance is reported where appropriate at a P value ≤ 0.05 with the Holm-Bonferroni adjustment for multiple comparisons.
Results
Larval response to temperature and insecticidal activity
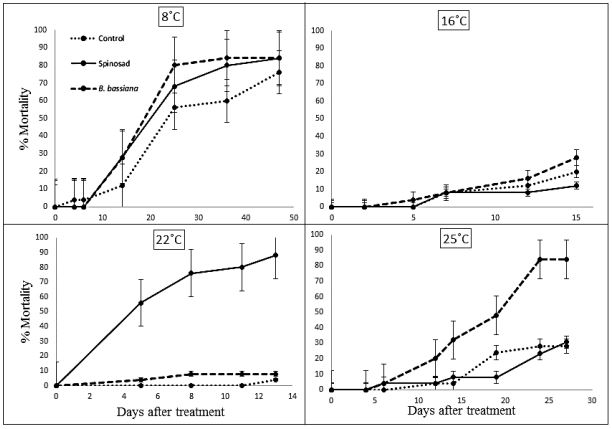
Temperature affected the efficacy of spinosad and B. bassiana on survival of T. confusum larvae. Insects exposed to low temperature (8 ˚C) sustained high rates of mortality (about 80% for control and both treatments) (Fig. 1). Biological control treatments were not significantly different from the control treatment at any time period during the experiment (F = 1.713; df = 2; P = 0.18). Mortality in the control was statistically equivalent to those with biological control agents throughout the 45 days of treatment. During this time, very few (< 10%) larvae reached the pupal stage.
Mortality at the intermediate temperature of 16 ˚C did not differ significantly between treatments (F = 0.658; df = 2; P = 0.52). Mortality slowly increased at the same rate across treatments over of the course of exposure at this temperature (Fig.1). Most larvae (> 60%) entered pupation by the end of 15 d, at which time the study was concluded.
Tribolium confusum at 22 ˚C experienced a high rate of mortality when exposed to spinosad (F = 62.53; df = 2; P < 0.001). After 14 d, larval mortality on spinosad approached 88% (Fig. 1).
Spinosad was significantly different from both B. bassiana and the control treatments (P < 0.001) as examined using Tukey’s HSD test for multiple comparisons. Beauveria bassiana and the control were not significantly different by post-hoc Tukey test. After 14 d, the majority (> 80%) of surviving larvae had entered the pupal stage.
At the highest temperature tested (25 ˚C), mortality was significantly higher in the B. bassiana treatment compared to control or spinosad (F = 24.21, df = 2, P < 0.001)(Fig. 1). The B. bassiana treatment resulted in over 80% mortality compared to < 10% mortality observed for the control and spinosad treatments. The experiment was allowed to run until all larvae were either dead or pupated. More than 80% of surviving larvae pupated in the first two weeks of this temperature treatment. All the dead larvae killed in the B. bassiana treatment yielded fungal hyphae indicative of B. bassiana when placed in chambers with high humidity to encourage fungal growth.
Adult t. Confusum response to B. bassiana
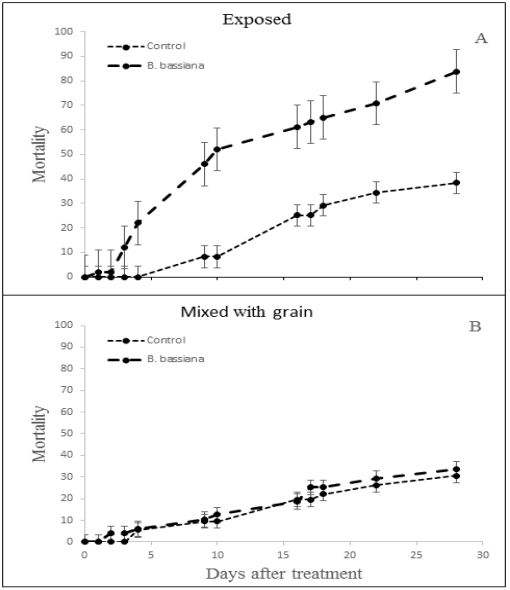
There were significant differences in adult response (Fig. 2) to B. bassiana under exposed and concealed environments (F = 5.035; df = 3; P = 0.002) at 22˚C. Beetles exposed to B. bassiana without grain were subject to high mortality (84 ± 8%), whereas beetles in the treatment that included grain showed similar mortality to the control (about 30 ± 10%) (F = 1.312; df =1; P = 0.254). All the dead beetles displayed fungal infection.
Acknowledgements
This work was funded by USDA-National Institute of Food and Agriculture Hatch (Accession# MONB00859) for funding. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA).
Fig. 1. Mortality of Tribolium confusum larvae exposed to Beauveria bassiana (large dash), spinosad (solid line) and control (small dash) at 8, 16, 22 and 25 C. Vertical error bars depict the residual SE of the mean.
Figure-2: Mortality of Tribolium confusum adults with exposure to Beauveria bassiana (large dash line) A) on paper and B) in grain compared with controls not exposed (small dashed line). Vertical error bars depict the residual SE of the mean.