Status of Sitodiplosis mosellana (Diptera: Cecidomyiidae) and its parasitoid, Macroglenes penetrans (Hymenoptera: (Pteromalidae), in Montana
Principal Investigator: Dr. Gadi V. P. Reddy.
Cooperators: Dr. Brian M. Thompson and Daniel E. Picard Western Triangle Agricultural Research Center, Montana State University,
9546 Old Shelby Rd, P.O Box 656, Conrad, MT 59425
Introduction
The orange wheat blossom midge, Sitodiplosis mosellana (Gehin) (Diptera: Cecidomyiidae), is a widespread pest of wheat of Palearctic origin that was introduced into North America in the early 1800s. This pest continues to expand to new areas of North America. The orange wheat blossom midge was first detected in northern and central Montana in 2000, near North Dakota where the midge had been detected earlier. Damage to the wheat crop in this region remained low, with only periodic minor outbreaks. Subsequently, an outbreak occurred in western Montana on spring wheat in the Flathead Valley. Initial estimates of wheat losses were over $1.5 million in Flathead County alone. Crop losses due to orange wheat blossom midge are often 30-80%, but can be100% if populations are extremely high. In North Dakota during 1990s, wheat growers lost an estimated $30 million in gross revenue due to S. mosellana. According to the published literature, losses in Canada exceeded US$38.65 million in Manitoba and US$77.30 million in Saskatchewan in 1995. In contrast, in eastern Montana where the midge’s parasitoid was found to be present along with the pest, significant losses were not reported.
In eastern Montana, adults of S. mosellana begin emerging from the soil between June and July. There is one generation per year and midges overwinter as mature larvae in the soil. Eggs are laid in developing wheat heads and larvae feed on the developing seeds, causing significant damage in spring wheat North America. Larval feeding on the emerging kernel causes shriveling and reduces the value of the crop. Wheat is vulnerable to attack by S. mosellana from the boot stage until anthesis is complete. Yield loss and broken kernels resulting from midge feeding reduce the grade of the harvested grain, lowering its value. The concealed feeding niche of the larvae under the wheat glume and the short flight period of the adults make this pest difficult to control with insecticides.
Natural enemies, where they are established, are important regulators of S. mosellana populations. Many hymenopteran natural enemies are reported to attack S. mosellana in either its native or introduced range. The main parasitoids of S. mosellana eggs and larvae in the wheat head are Macroglenes penetrans (Kirby) (Hymenoptera: Pteromalidae), Platygaster sp., (Hymenoptera, Platygastridae) and Euxestonotus error (Fitch) (Hymenoptera, Platygastridae).
All these parasitoids was introduced to North America shortly after the arrival of S. mosellana and are still a valuable tool in maintaining the population level below the threshold level. In addition, native Ground beetles (Coleoptera: Carabidae) are known to feed on midge larvae on the soil surface just before they enter winter diapause refuge in the soil in the fall and again in the spring before pupation and adult emergence. While specialist and generalist natural enemies both have potential to reduce wheat midge populations, their efficacy can be variable, either to due local environmental factors or, for the introduced natural enemies, the history of their spread, either tracking or not, the spread of the midge to new areas.
Macroglenes penetrans is the most important natural enemy of S. mosellana. It was introduced from Europe to Canada as part of a classical biological control program against wheat midge. In Canada, M. penetrans plays an important role in controlling S. mosellana populations, parasitizing up to 80% of the midge population yearly. In Montana, efforts to achieve biological control of wheat midge began in 2008 using M. penetrans introduced from North Dakota to Kalispell, around Flathead, Montana (B. Stougaard, Personal communication). From 2008 to 2014, no evidence of establishment of the parasitoid in Kalispell was found and a second attempt was made, using a second population of the parasitoid from Alberta. Approximately 700 M. penetrans were released in each of Pondera and Flathead Counties (Kalispell) in July of 2014.
The total of 1400 M. penetrans in both of these some general region in western Montana.
In the wheat plant, larval S. mosellana feed on the developing wheat seed in a protected environment under the glume of the wheat seed. This concealed feeding niche protects the midge larvae from both parasitoids and pesticides, making timing of spraying critical for success. The ability of parasitoids to actively target their host, to synchronize their emergence with that of S. mosellana and to respond to changes in host populations makes them an attractive alternative to chemical control. The parasitoid M. penetrans is well synchronized with wheat midge larvae and able to reach them at their feeding sites. It is estimated to control anywhere from 40-80% of the
S. mosellana population. While potentially able to limit damage by wheat head midge, efficacy of the parasitoid M. penetrans may fail either because of poor synchrony of adult parasitoids in a given area with the necessary host stage or because the pest has dispersed geographically into regions not yet occupied by the parasitoid. Host parasitization is dictated by the ability of the parasitoid to find prey at both long and short distances.
The pest, S. mosellana, is a weak flyer that primarily locates susceptible wheat at short range via plant volatiles. The movement and establishment of the wheat midge into new areas is therefore driven by movement of air masses during brief weather events. Recently, wheat midge has colonized wheat growing regions of western and central Montana (in 2006 and 2012, respectively) for the first time. This eruption of the pest in new wheat-growing regions has led many to speculate that the pest has left behind natural control factors like its parasitoid, M. penetrans. However, additional factors also affect S. mosellana population dynamics.
Precipitation, either through rainfall or irrigation, plays an important role in midge emergence from the soil, as does the total available temperature. Differences in emergence and the timing of emergence under irrigated versus rain-fed conditions have not yet been evaluated. Moisture does, however, interact with insect emergence from the soil and from wheat heads before entering the soil and winter diapause. Larval emergence from wheat heads usually coincides with a summer rainfall, presumably because it loosens the soil, facilitating larval entry into the soil.
Conventional pesticide management of wheat midge recommends treatment if there is one adult midge for every 4–5 wheat heads during heading. Several insecticides have been found to be effective and can be applied to wheat at heading for S. mosellana control. Currently, the most common method of control is an application of chlorpyrifos (Lorsban™) or lambda-cyhalothrin (Warrior®), timed to kill adult S. mosellana during their peak emergence. Peak emergence typically occurs over only a few days and each adult has a short life span. Thus, timing is very important but is difficult to accurately determine using pheromone traps. Furthermore, prolonged emergence in irrigated fields may expose new shoots after the 2-5 day activity of the pesticide has passed.
The incorporation of parasitism by M. penetrans into an integrated pest management program may allow for greater control while lowering the need for pesticide applications, which can harm non-target beneficial insects in the crop like the wheat stem sawfly parasitoids Bracon cephi (Gahan) and Bracon lissogaster Mues. (Hymenoptera: Braconidae), which have the same flight season as S. mosellana. Carabid ground beetle predators of S. mosellana are another important group of wheat midge natural enemies found in Montana wheat fields that may be harmed by pesticide applications. Late insecticide applications can actually prove detrimental to pest control by reducing certain populations of these parasitoids.
The present study was undertaken to determine the prevalence and dispersal rate of S. mosellana and its dominant parasitoid M. penetrans in the central Montana. This study examined the pest population and the prevalence of its natural enemy M. penetrans in central Montana, the area most recently colonized by S. mosellana. The study was undertaken by using three approaches, adults in pheromone traps, larvae in wheat heads, and larvae + cocoons in soil in winter. The objectives were (1) To see if wheat head midges are more abundant in dryland vs irrigated wheat; (2) To see if parasitoids of wheat head midge are more abundant in dryland vs irrigated wheat; and (3) To see if different measurements of wheat head midge abundance are correlated or not.
Materials and Methods
Study Area
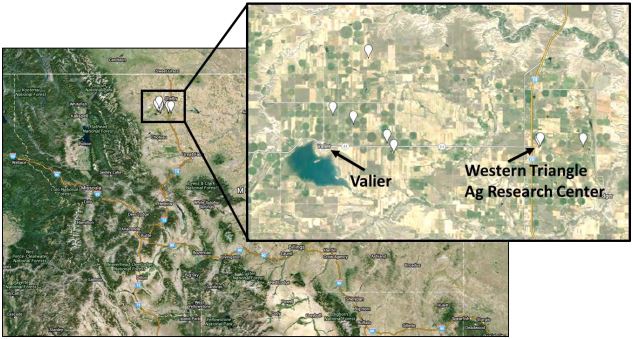
Surveys for this study were carried out in central Montana, in the area around Valier, a wheat producing region known as the “Golden Triangle.” Sitodiplosis mosellana was first documented in the study region in 2012. Work here was done in 2014 and 2015. Since 2012, wheat fields in the area have had persistent populations of S. mosellana. Wheat in the area is produced either as dryland or irrigated wheat, in rotation with canola, peas, or barley. The dominant crops in the region are spring and winter wheat. However, S. mosellana is pest on spring wheat in Montana. Surveys were conducted from May 10th – July 31st 2015 in spring wheat to span the flight season of S. mosellana and M. penetrans. The 33 sites were selected and marked those on the map in Fig. 1. In each of the 8 areas, pre-existing growers’ fields that vary in size were chosen and established in the ~230 sq km wheat-producing area surrounding Valier, Montana (Fig. 1). Midge abundance in each field was estimated in four different ways: (1) pheromone traps for adult midges (in 2014), (2) sweep net sampling for adult parasitoids, (3) counts in wheat seed head for midge larvae, and (4) counts in soil cores for midge larvae or cocoons and associated parasitoids. Both dryland and irrigated spring wheat fields were sampled in roughly equal numbers.
Pheromone trapping of adult midges, 2015
Midge populations were surveyed in 2015 using delta traps baited with a pheromone lure (2,7- nonanediyl dibutyrate) (Great Lakes IPM, Inc., Vestaburg, MI 48891) with sticky card inserts (Scentry®) at all of the 34 study sites (Table 1) Delta traps were painted green to reduce non- target catch and positioned at the height of the wheat canopy. Each trap was placed 20 m from the field edge. The trap height was adjusted weekly to match the height of the wheat canopy. The midge pheromone used attracts only male S. mosellana. Therefore, total midge population was calculated using documented sex ratios and average flight potential for male midges. Because sex ratios for S. mosellana are typically 1:1 across most populations, and because both male and female S. mosellana typically fly less than 500 m, we estimated local minimum female numbers near each trap from male trap catch by assuming this sex ratio and a flight distance of 400 m.
Wheat head infestation rates
Wheat heads were sampled before the current year S. mosellana larvae began dropping to the soil (July 31st – August 4th 2015). We sampled 34 locations for the presence of S. mosellana in soil within and in the vicinity of the Western Triangle Agricultural Research Center (Table 1).
Estimates per wheat head were based on examining 200 wheat heads that were randomly selected and marked off the fields in a grid at each of the 34 sites and placed in mesh linen bags. These wheat heads were taken to the laboratory and dried under low humidity at room temperature before individually threshing wheat heads by hand to extract larvae S. mosellana.
The average number of wheat heads per square meter was measured in each study site by randomly placing a square meter quadrat in three haphazard locations in each field and counting the total number of wheat stems post-harvest. The percentage of wheat infested out of the 200 randomly sampled wheat heads calculated and multiplied by our estimate of stems/m2 to estimate larvae in wheat heads/m2.
Densities of Wheat head midge overwintering stages in soil
Overwintering S. mosellana populations in soil (larvae+cocoons) were sampled in the fall of 2014 (n = 12) and spring of 2015 (n = 22) using a 5.7 cm dia bulb planter, coring to a depth of
7.6. Soil samples were selected along with transect starting 30 m from the edge the field, taking 10 samples per transect, with samples spaced 10 or more m apart. Each field was sampled only once, with only one transect. Soil samples were held at 6˚C until S. mosellana larvae and cocoons were separated from inorganic material using a soil washer (Flote-Tech Model A, R.J. Dausman Technical Services Inc., Rochester, Indiana, USA), where the organic material was gently broken apart from inorganic material water movement and air bubbles. Organic debris, including S. mosellana stages, was routed to a fine collection screen where larvae and cocoons were collected with forceps and placed in alcohol for examination and counting.
Parasitoid sweep net survey
Adult M. penetrans parasitoid abundance was estimated concurrently with sampling of wheat heads for S. mosellana larvae via sweep netting at all sample locations. Each sample consisted of 20 sweeps (180°) along a haphazardly directed line across the sample field within 400 m of the S. mosellana pheromone traps. Sweep net samples were taken once a day, five days per week, from wheat heading through anthesis, from June 1st - July 30th 2015. Adult parasitoids similar to M. penetrans were collected from field samples and were then identified in the laboratory using reference specimens and counted. Surveys were conducted at eight locations within Pondera County, MT (same locations as shown in Figure 1).
Data analysis
Abundances of wheat head midges and their parasitoid M. penetrans were compared in irrigated vs. dryland cropping systems using t-tests, for all of them. Sex ratios were calculated for irrigated and dryland M. penetrans populations. Phenology diagrams for male wheat head midges (pheromone traps) and both sexes of the parasitoid (sweep net samples) were constructed from sample data and the 10, 50 and 90 percentile points for emergence were calculated in relation to a scale of cumulative degree days (above 5 ˚C) starting January 1 of each year. Daily precipitation values were obtained for the study area using the USDA-NRCS weather station located at Western Triangle Agricultural Research Center (N 48.307404, -W111.921977 Lat. Long.). Correlations between midge populations in different locations were evaluated using linear models in the statistical package R. Data were log transformed where necessary to maintain model assumptions.
Results
Pheromone trap catch of adult midges, 2014
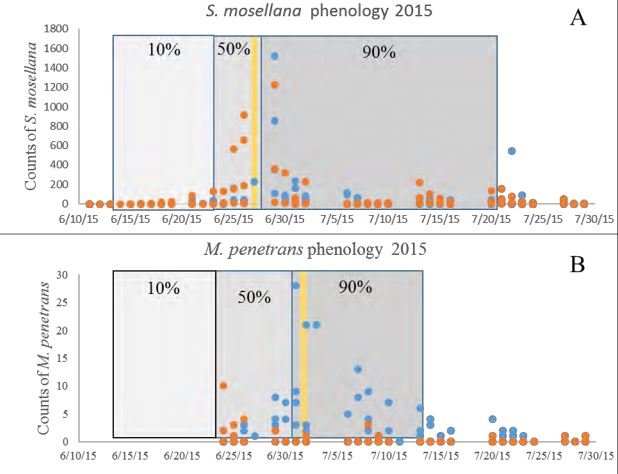
Summed over all 34 sites in the study area, phenology of wheat head emergence (Fig 2) showed that the first S. mosellana adults in 2014 were collected 16 June in the study area. By 23 June 23, 10% of S. mosellana had emerged (8.5 ± 1 days; mean ± SE) and 752 degree days had accumulated at or above 5˚C since 1 January. Fifty percent of S. mosellana had emerged by 26 June 26 (11.7 ± .5 days after first midge emergence) at 1,045 degree days. On 30 June (1,453 degree days) 90% of S. mosellana had emerged (25 ± 2.5 days after firstcollection). A few additional S. mosellana adults continued to emerge throughout July, well after the majority of the crop had progressed past susceptibility (boot – early heading). The emergence of S. mosellana followed a pattern of a large initial emergence peak followed by a long tail of declining trap captures throughout the season (Fig. 2A). In 2015, peak emergence occurred at all survey locations on 29 June (1,149 degree days). Late maturing wheat tillers were present throughout the flight season of S. mosellana in this study. The relative importance of infestation of tillers to main stems in the population dynamics of S. mosellana was not examined in this study.
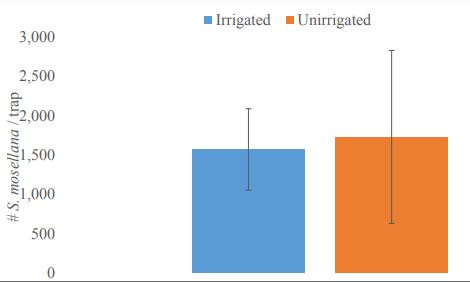
Pheromone traps caught a total of 10,918 male S. mosellana from all study locations. The populations of S. mosellana were not significantly (P <0.05) different between irrigated and unirrigated cropping systems (1,500 ± 500 and 1,700 ± 1,100, respectively; mean ± SE) (t = - 0.131, df = 2.91, P= 0.9043; Figure 3). Trapping area was estimated to be up to 1,600 m2 (0.16 hectares) based on the flight potential of the male midge. The calculated midge population per hectare, assuming an even sex ration, was therefore 18,100 ± 5,300 midges (of both sexes) per hectare The estimated egg load for a population of this size, and thus the damage potential assuming a mean of 80 eggs/female is 724,200 ± 211,500 eggs/hectare. This number represents the maximum number of wheat seeds that could be infested per hectare assuming each egg is laid on a separate seed.
Wheat head infestation rates
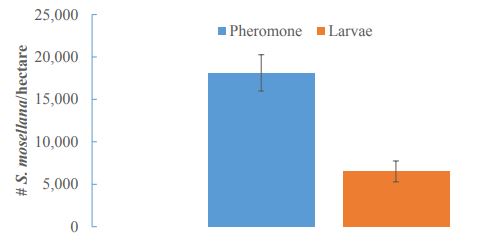
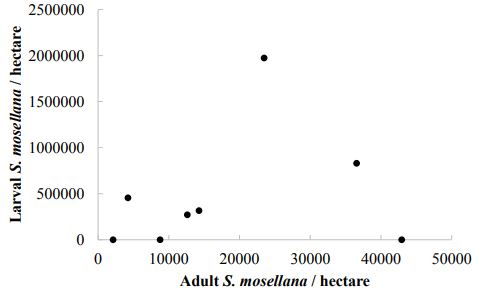
Counts of S. mosellana larvae in wheat heads yielded 53 ± 24 midges per m2, which amounts to 549,200 ± 260,700 S. mosellana larvae per hectare. The average egg load of a female S. mosellana is 80 eggs. Larval counts therefore suggest an average of 6,865 females per hectare. Pheromone traps instead predicted an average of 9,000 female S. mosellana (a 24% difference). Estimates of populations by larvae in wheat heads and by pheromone traps were not significantly different, although they were very nearly significant (t = 2.2073, df = 9.38, P= 0.05352; Figure 4). Differences in population estimates likely stem from pheromone traps having a trapping distance greater than the 400 meters assumed for this study. Accounting for the 30% difference in trapping from larval counts equates to an effective trapping area of 0.21 hectares for the pheromone traps used in this study. There was no correlation between the number of S. mosellana larvae found in wheat heads per hectare and the number of S. mosellana adults caught in pheromone traps across all sampled fields (Figure 5).
Densities of wheat head midge overwintering stages in soil
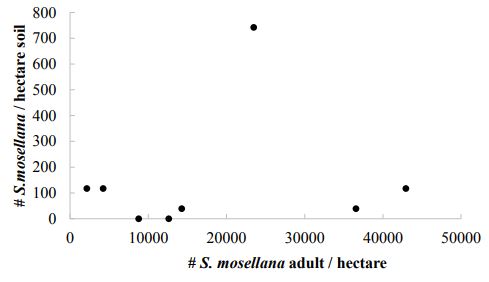
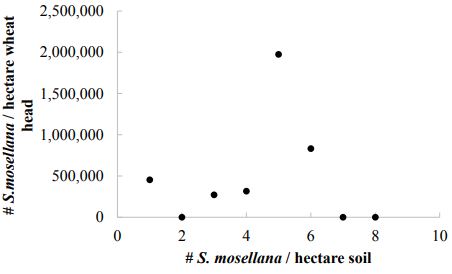
Larvae of S. mosellana and cocoons represent the potential population of S. mosellana in the approaching season and are used to predict midge pressure in some regions (Jacquemin et al. 2014). Despite a higher concentration of S. mosellana in the soil of irrigated cropping systems, differences were not significantly different between irrigated and unirrigated systems (t = - 1.5155, df = 18.847, P= 0.1463; Figure 6). The number of S. mosellana sampled from soil did not correlate with the number of adult S. mosellana in pheromone traps (Figure 7) nor the number of larvae in wheat heads in this study (Figure 8). Soil sampling predicts 636,100 ± 219,600 midge/hectare (mean ± SE), in contrast to the 13,600 and 18,100 midge/hectare predicted by end of season larval counts and pheromone traps (respectively).
Macroglenes penetrans survey
Macroglenes penetrans sweep net catch peaked shortly after peak capture of wheat head midges in pheromone traps. (Figure 2B). In 2015, parasitoid peak emergence occurred on the 2 July (1,235 degree days) around Valier, MT. Meanwhile, the M. penetrans flight period lasted from 23 June 23 – 31 July. Ten, fifty and ninety percent population emergence occurred 3.4±1.3, 17.5±0.6 and 31.3±11.9 SE days after the first parasitoid was captured in a given field (26 June 26, 1 July, and 14 July 2015. There was no correlation between the number of S. mosellana adults captured in pheromone traps in a wheat field and the number of M. penetrans adults captured in sweep nets (F1,6 = 0.147, P = 0.715, r2 = 0.14).
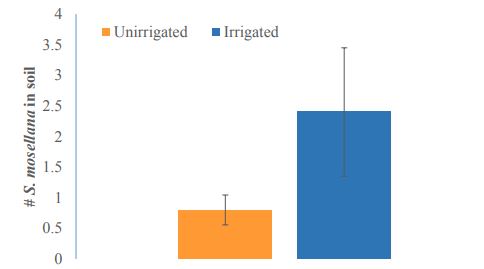
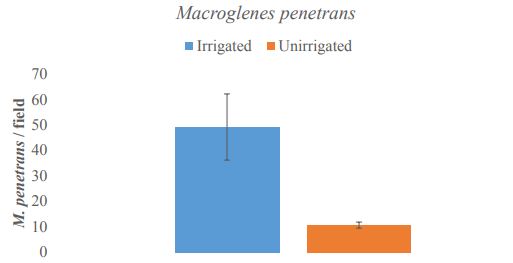
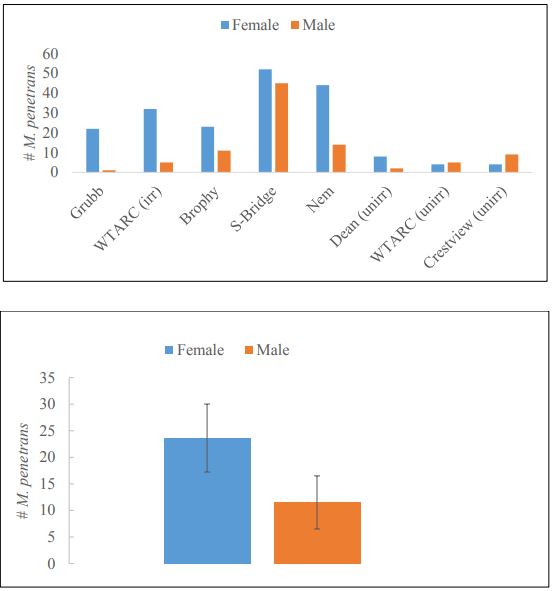
Irrigation significantly increased the abundance of M. penetrans in wheat fields compared to unirrigated cropping systems (t = 2.8891, df = 4.065, P= 0.04375) (Figure 9). Irrigated fields had on average five times as many M. penetrans as unirrigated cropping systems (49.2 ±13 and 10.6±1.2, respectively). Meanwhile, the sex ratio of M. penetrans varied between sampling locations. Despite an apparent bias toward female parasitoids (female M. penetrans outnumbered males 2:1 overall; Figure 9A), differences were not statistically significant in this study (t = 1.4861, df = 13.248, P= 0.1607), and at individual study locations the ratio of male to female M. penetrans varied greatly (Figure 10B).
Acknowledgements
This work was supported by the Montana Wheat and Barley and USDA-NIFA, Multistate Project W3185, The Working Group Biological Control of Pest Management Systems of Plants [Accession number# 231844]. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We thank Dr. Héctor A. Cárcamo (Agriculture and Agri-Food Canada), Scott Meers (Alberta Agriculture and Forestry) and Brooke Bohannon (MSU Northwest Ag Research Center) for participating in the exploratory trip in Canada.
Table-1: Density of Sitodiplosis mosellana larvae + cocoons (L+P) in soil /m2 in winter of 2014- 2015and pheromone catch of male S. mosellana adults in each field in 2015.
|
Site name |
Type |
L+P/m2 mean± SD |
adults/trap mean ± SD |
|
Mageris |
Unirrigated |
0±0 |
249±7.6 |
|
Sunburst |
Unirrigated |
0±0 |
- |
|
MFE |
Irrigated |
39±3.4 |
2,524±24.3 |
|
Sil Farm |
Irrigated |
78±2.8 |
- |
|
Akin |
Irrigated |
0±0 |
244±3.6 |
|
Birch |
Irrigated |
0±0 |
289±6.6 |
|
Wingina |
Unirrigated |
0±0 |
519±4.3 |
|
Fretheim |
Irrigated |
0±0 |
267±4.2 |
|
Banka W |
Unirrigated |
39±2.6 |
15±4.3 |
|
GC |
Unirrigated |
78±6.2 |
101±2.8 |
|
JVD chucks |
Irrigated |
195±4.7 |
366±7.3 |
|
Banka E |
Unirrigated |
39±2.2 |
62±2.6 |
|
Crawford |
Irrigated |
156±6.3 |
1±0.5 |
|
Banka #1 |
Unirrigated |
0±0 |
499±8.6 |
|
Banka #2 |
Unirrigated |
78±4.1 |
40±2.1 |
|
Banka #3 |
Unirrigated |
78±3.6 |
62±6.6 |
|
Banka #4 |
Unirrigated |
117±2.8 |
15±2.9 |
|
Hoss |
Irrigated |
0±0 |
1±0.3 |
|
Parm |
Unirrigated |
39±6.3 |
5±2.4 |
|
Wheeler |
Unirrigated |
0±0 |
85±8.7 |
|
Moon |
Irrigated |
0±0 |
4±2.1 |
|
Valley |
Irrigated |
0±0 |
2±1.7 |
|
Section |
Unirrigated |
0±0 |
274±6.6 |
|
Hunter |
Irrigated |
0±0 |
9±2.2 |
|
Dewey |
Irrigated |
39±3.4 |
1±0.4 |
|
Turk |
Irrigated |
39±4.8 |
6±1.8 |
|
Webster |
Irrigated |
156±8.2 |
2±0.7 |
|
KB Stark |
Irrigated |
742±8.6 |
28±1.3 |
|
Dean #2 |
Unirrigated |
0±0 |
39±4.1 |
|
Pondera5 |
Irrigated |
117±4.4 |
2644±22.3 |
|
WTARC1 |
Irrigated |
117±6.3 |
750±11.4 |
|
WTARC2 |
Unirrigated |
0±0 |
249±6.5 |
|
KB #2 |
Irrigated |
39±4.1 |
244±7.2 |
|
Casey |
Unirrigated |
39±2.7 |
245±9.2 |
Figure-1. Sitodiplosis mosellana and parasitoid (Macroglenes penetrans) sampling locations (white markers) in Central Montana 2015.
Figure-2. Flight phenology of Sitodiplosis mosellana (A) (male only) and Macroglenes penetrans (B) (both sexes) in Pondera County in 2015 in irrigated (blue) and unirrigated (orange) cropping systems. Gray areas denote the percentage of population emerged and the yellow bar denoted the peak emergence date.
Figure-3. Sitodiplosis mosellana abundance in irrigated (blue) and unirrigated (orange) cropping systems (t = -0.131, = 0.9043).
Figure-4. Sitodiplosis mosellana populations as predicted by pheromone baited trap and by counts of larvae in wheat heads (Larvae) were marginally significantly different by t-test (t = 2.2073, df = 9.38, P= 0.05352).
Figure-5. The number of Sitodiplosis mosellana larvae found in wheat heads per hectare does not correlate with the abundance of adults caught in pheromone traps in the same field in 2015.
Figure-6. Sitodiplosis mosellana larval populations sampled from soil in 2015 in unirrigated (orange) and Irrigated (blue) cropping systems.
Figure-7. The number of Sitodiplosis mosellana per hectare in the pheromone traps was independent of the number of midges in the soil in the same field.
Figure-8. The number of Sitodiplosis mosellana estimated per hectare is independent of the number of larvae removed from wheat heads in the same fields.
Figure-9. Macroglenes penetrans abundance in irrigated (blue) fields was significantly higher than in unirrigated (orange) cropping systems (t = 2.8891, df = 4.065, P= 0.04375). * indicates p< 0.05 significance level.
Figure-10. The sex ratio of female (blue) to male (orange) of Sitodiplosis mosellana in sweep net samples of wheat fields in the Valier area at each individual field (A) and combined across all fields (B). Sex ratios were often highly biased at individual locations, but were not significantly different across all fields (t = 1.4861, df = 13.248, P= 0.1607).