Toxic effects of biologically derived insecticides on larvae of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae)
Principal Investigator: Dr. Gadi V. P. Reddy.
Cooperators: Dr. Frank B. Antwi, Dr. Takashi Kuriwada
Western Triangle Agricultural Research Center,
Montana State University,
9546 Old Shelby Rd,
P.O. Box 656,
Conrad, MT 59425
Introduction
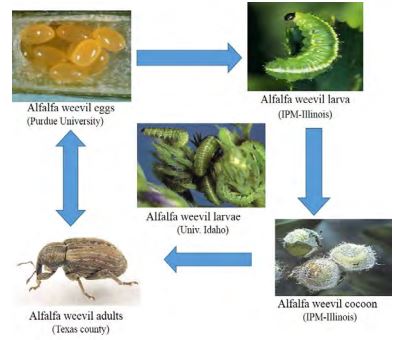
Alfalfa weevil, Hypera postica (Gyllenhall) (Coleoptera: Curculionidae), is the most destructive insect pest of alfalfa hay in the intermountain west of the United States. Hypera postica not only decreases yield and quality of the first cutting, but can also harm subsequent cuttings. Both larvae and adults damage terminals, foliage and new crown shoots, thereby lowering crop yield and quality. However, larvae cause the most damage (Figure-1). During severe infestations, larvae can cause substantial defoliation, resulting in severe first cutting losses. Heavily infested fields may appear silver or white, as most leaves are skeletonized or consumed entirely. If large numbers of adults or larvae survive until harvest, they can damage stems and crown buds, retarding regrowth. A decrease in stem elongation occurred at a density of 30–100% of the smallest larval density.
Residual effects from severe damage decrease plant vigor, resulting in lower stand density and poor yields in subsequent harvests.
Figure-1: Life Cycle of the Alfalfa Weevil
Although H. postica is native to Europe it was inadvertently introduced into the western United States in the early 1900s and again, separately, into the eastern United States in the late 1940s. In Montana, alfalfa (Medicago sativa L., Fabaceae) is the second most important crop after small grains. Alfalfa growers in Montana first began to notice H. postica during spring 2013 when the weevil caused considerable damage and yield losses. In addition, alfalfa weevils caused economic damage in irrigated fields in the Yellowstone and Missouri river valleys in Montana. As a rule, insecticidal treatment are considered economical when larval populations average between 1.5 – 2.0 larvae / stem, or 20 larvae / sweep. In 2014 and 2015, an H. postica outbreak occurred in Valier, Montana. Even though H. postica does the most damage before the first cutting, considerable damage was noticed even after the first harvest.
To date, other than classical biological control, insecticide applications and early harvesting are the most common management strategies for alfalfa weevil. However, most of the chemical insecticides used to control this pest are extremely hazardous to bees and other beneficial insects like the parasitoids Bathyplectes curculionis (Thomson) (Hymenoptera: Ichneumonidae) and Oomyzus incertus Ratzburg (Hymenoptera: Eulophidae). Increasing concerns for environmental safety and insecticide resistance arising from frequent use of synthetic insecticides affect the long-term feasibility of the current strategy of alfalfa weevil management. Consequently, many alfalfa growers in north central and central Montana are looking for more environmental friendly control methods for managing this destructive pest.
Several reports have demonstrated the effectiveness of entomopathogenic fungi against many species of beetles and weevils. Beauveria bassiana and Metarhizium anisopliae sensu lato (formerly M. anisopliae) was been reported to infect larvae and adults of various weevil species and have been used successfully in biological control experiments against these pests. Hypera postica was reported to infect B. bassiana. In addition, it has been reported that high mortality of H. postica caused by M. brunneum in Japan.
Alfalfa is a favorable habitat for many beneficial arthropods including pollinators and natural enemies of pests (Nicholls and Altieri, 2013). A number of naturally derived insecticides have been reported to be safe to many types of non-target organisms. Here, we studied the effects of several commercially available, biologically derived insecticides against larvae of H. postica.
Materials and methods
Rearing of Insects
Hypera postica larvae were collected from alfalfa fields in Valier, Montana, USA, using sweep nets in July, 2015 and taken to the laboratory. Larvae were placed in collapsible cages (12 × 10 × 10 cm), fed alfalfa foliage, and held at 22 ± 2 °C, 70–80% r.h. and an approximately14:10 h L:D photoperiod. Field-collected larvae were separated by instar as described by Harcourt (1981). The instars ranged from first to fourth instars. The first instar is light yellow or tan in color with a darker head and about 1 mm long while the second instar is yellowish-brown with their head deepening to black, third and fourth instar size is up to 9 mm long, are bright green with shiny black head capsule, and have a white stripe down the halfway point of their rears. Second instars were used for all tests.
Insecticides
Insecticides tested were commercial formulations (Table 1) stored dry place at 4-5 °C until diluted them to the desired concentrations for use. Concentrations tested were 0.1, 0.5, 1.0 and 2.0 times the lowest label rate, except for Entrust, which had additional concentrations of 0.001 and 0.01 times the lowest label rate also prepared due to the high toxicity of this compound.
Toxicity tests
Toxicity tests were carried out in the laboratory from 15 July through August 2015 when test larvae from field populations were available. Materials were applied via contact at the desired concentrations (see Table 1 for rates). For each replicate, five larvae were transferred onto a disk of Whatman No. 1 filter paper (9 cm diam, Whatman® quantitative filter paper, ashless, Sigma-Aldrich, St. Louis, Missouri, USA) in a 9 cm disposable Petri dish where they were topically treated with the test material.
Each Petri dish also contained three alfalfa stems about 5 cm long, each with 6-8 leaves as larval food. Six replicate Petri dishes, containing a total of 30 larvae, were treated (using a Sprayer (Sprayco, Livonia, MI) with 1 mL of a test material (Reddy et al., 2014). Controls were treated with 1.0 mL of tap water. Following application, Petri dishes were held under the same laboratory conditions used for rearing, and larval mortality was assessed daily for nine days.
Statistical analyses
Because mortality rates for several of the tested materials (Mycotrol, Met 52, Aza-Direct, Xpulse, Xpectro and Entrust, Figs. 1-6) increased over time, treatments were analyzed with probit analysis using the program Probit-MSChart (http://140.120.197.173/ecology/ Download/Probit-MSChart.rar). Control mortalities were adjusted using the Abbot method (Abbott, 1925). Mortality response (in probits) was regressed against log10 day.
Results
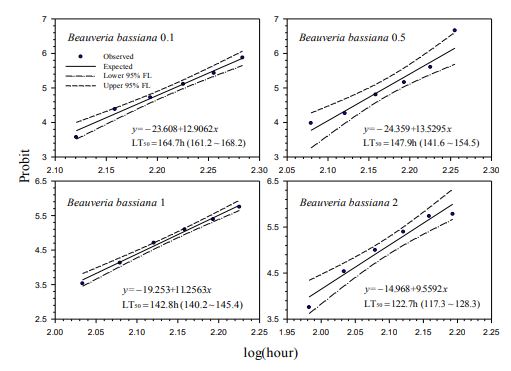
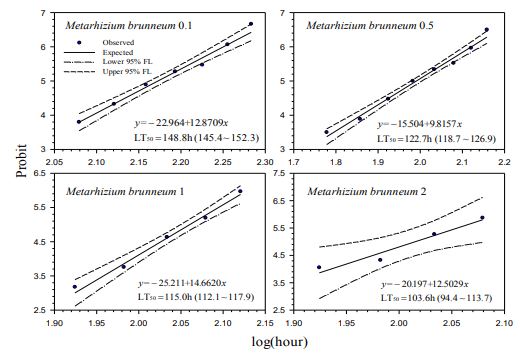
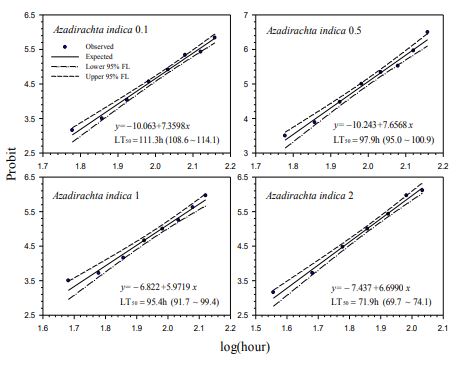
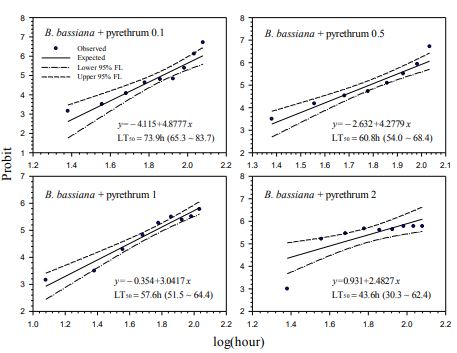
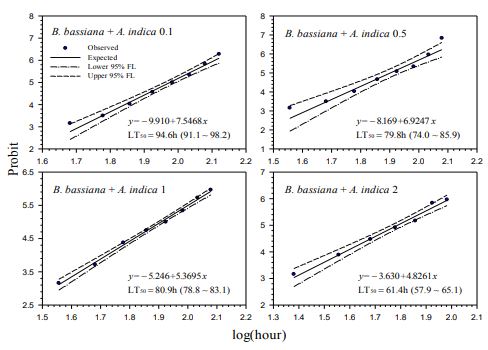
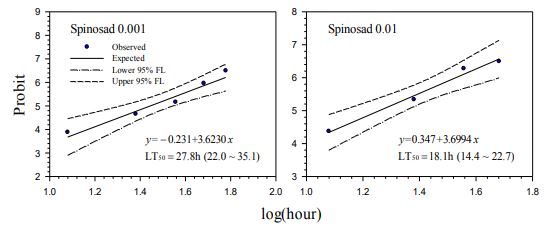
Our contact bioassays for H. postica found a good linear regression relationship between mortality (in probit) of H. postica and time (log10 day) after treatment with Mycotrol, Met52, Aza-Direct, Xpulse, Xpectro and Entrust (Figs. 2-6). The mortality rate (in probit) increased with log10 day for Mycotrol, Met52, Aza-Direct, Xpulse, Xpectro and Entrust. For Mycotrol (Beauveria bassiana), the mortality responses for a unit change in time (log day) varied from 9.56 to 13.53 probit units (Fig. 2). For Met 52 (Metarhizium brunneum), mortality rates (probit) for a unit change in log10 hour (log10 day) ranged from 9.82 to 14.66 probit units (Fig. 3). For Aza-Direct (azadirachtin), probit units varied from 5.97 7.66 for a unit change in log10 hour (log10 day) (Fig. 4). For Xpectro (Beauveria bassiana + pyrethrin), probit units ranged from 2.48 to 4.88 for a unit change in log10 hour (log10 day) (Fig. 5). For Xpulse (Beauveria bassiana + azadirachtin), probit units for a unit change in log10 hour (log10 day) varied from 4.83 to 7.55 (Fig. 6). For Entrust, probit units ranged from 3.62 to 3.7 for a unit change in log10 hour (log10 day) (Fig. 7).
In the treatment of H. postica with B. bassiana (Mycotrol) the LT50 ranged from 122.7 to
164.7 hours (5.11 to 6.86 days) (Fig. 1). For M. brunneum (Met52) the LT50 ranged from
103.6 to 148.8 hours (4.32 to 6.2 days) (Fig. 2). The LT50 for A. indica (Aza-Direct) ranged from 71.9 to 111.3 hours (2.996 to 4.64 days) (Fig. 3). The LT50 for B. bassiana + pyrethrum (Xpectro) ranged from 43.6 to 73.9 hours (1.82 to 3.08 days) (Fig. 4). For (B. bassiana + A. indica) Xpulse the LT50 ranged from 61.4 to 94.6 hours (2.56 to 3.94 days) (Fig. 5). Finally, for Entrust (spinosad) all larvae died within 12 hours of treatment (data not shown) for the concentrations 0.1 to 2.0. However, LT50 for the lower concentrations (0.001 and 0.01) ranged from 18.1 to 27.8 hours (Fig. 6), making Entrust the most toxic of the products tested. Generally, LT50 decreased with increasing time log10 hour (log10 day) for all treatments and concentrations (Figs. 1-6).
Acknowledgments
This work was supported by USDA National Institute of Food and Agriculture, Multistate Project W3185, The Working Group Biological Control of Pest Management Systems of Plants [Accession number# 231844]. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We also thank Dr. Hsin Chi for the help with statistical analysis of the data.
Table 1 Materials and application rates used for the laboratory bioassays against Hypera postica larvae.
|
Treatment |
Chemical name |
Trade name |
Concentrations (ml/L) |
Source |
|
T1 |
Untreated control |
- |
- |
- |
|
T2 |
spinosad (Saccharopolyspora spinosa) |
Entrust® a wettable powder |
0.000091, 0.00091, 0.0091, 0.0455, 0.091, and 0.182 |
Dow Agro Science LLC, Indianapolis, IN |
|
T3 |
Metarhizium brunneum F52 |
Met52® emulsifiable concentrate |
0.072, 0.36, 0.72, and 1.44 |
Novozymes Biologicals, Salem, VA |
|
T4 |
Beauveria bassiana GHA |
Mycotrol ESO® emulsifiable concentrate |
0.072, 0.36, 0.72, and 1.44 |
LAM International, Butte, MT |
|
T5 |
Azadirachtin (extracts from Azadirachta indica) |
Aza-Direct® emulsifiable concentrate |
0.144, 0.72, 1.44, and 2.88 |
Gowan Company, Yuma, AZ |
|
T6 |
B. bassiana GHA + pyrethrins |
Xpectro® emulsifiable dispersible oil |
0.25, 1.25, 2.5, and 5.0 |
LAM International, Butte, MT |
|
T7 |
B. bassiana GHA + cold pressed Neem extract |
Xpulse® emulsifiable dispersible oil |
0.072, 0.36, 0.72 and 1.44 |
LAM International, Butte, MT |
Figure-2. Probit analysis of lethal time of Beauveria bassiana to Hypera postica at different concentrations.
Figure-3. Probit analysis of lethal time of Metarhizium brunneum to Hypera postica at different concentrations.
Figure-4. Probit analysis of lethal time of Azadirachta indica to Hypera postica at different concentrations.
Figure-5. Probit analysis of lethal time of Xpectro® (B. bassiana GHA + pyrethrins) to
Figure-6. Probit analysis of lethal time of Xpulse® (B. bassiana GHA + azadirachtin) to
Fig. 7. Probit analysis of lethal time of Entrust® (spinosad) on to Hypera postica at different concentrations.