Evaluation of the effectiveness of entomopathogenic fungi, reduced risk chemicals, and trap crops for the management of wireworms on spring wheat
Principal investigator: Dr. Gadi V.P. Reddy1
Project collaborators: Stefan T. Jaronski2, Kevin Wanner3 and Heikki M. Hokkanen4
Project personnel: Anamika Sharma1, Shabeg Briar1, Ramandeep Kaur Sandhi1 and John H. Miller1
1Western Triangle Agricultural Research Center, Montana State University, Conrad, MT
2United States Department of Agriculture, Agricultural Research Service, Northern Plains Agricultural Research Laboratory, 1500 N. Central Avenue, Sidney, MT
3Department of Plant Science and Plant Pathology, Montana State University, Bozeman,
3Department of Plant Science and Plant Pathology, Montana State University, Bozeman,
4Department of Agricultural Sciences, University of Helsinki, FI-00014 Helsinki, Finland
Aim of the study
The aims of this study were: 1) to evaluate the effectiveness of trap crops for the management of wireworms and 2) to evaluate efficacy of entomopathogenic fungi for wireworm management under lab and field conditions.
Figure. 1. Wireworm damage to a spring wheat plant in Golden Triangle Area, Montana.
Material and Methods
Experimental sites
In earlier studies (Reddy et al. 2014; Adhikari and Reddy 2017), highly wireworm infested fields were selected in Ledger and Valier in the ‘Golden Triangle’ area of Montana. In 2017, experiments were conducted at two growers places Ledger (N48°18’26.9244 W111o51’34.4376) and Valier (N48°18’37.4148 W112°25’19.0956). Experiment plots were seeded on 4 May 2017 in Valier and 10 May 2017 in Ledger. Before seeding, the herbicide glyphosate (RT3®, Monsanto Company, St. Louis, MO) was applied at the rate of 2.5 L/ha for weed control, following regional farming practice. Fertilizer (N, P, and K) was applied at a ratio of 224.2, 0, and 22.4 kg/ha by broadcast application during planting, and an additional fertilizer application (N, P, and K at a ratio of 12.3, 25.2, and 0 kg/ha) applied through the seed plot drill. The experimental plots received 5 cm of water via overhead irrigation whenever needed. The first irrigation was done 30 days after treatments. Harvesting was done at in July 2017.
Sampling for plant damage and wireworm density
To determine the level of crop damage from wireworms, the number of plants or seedlings in each plot was measured randomly using the 1 m line intercept method. From an individual plot two count are taken each from wheat and trap crops. The first count are taken two weeks after sowing. The subsequent counts are taken at the interval of a week in both sites. The same method was applied to the various trap crops intercropped with the wheat. The relative effectiveness of the various trap crops in attracting wireworms will be assessed through soil core samples taken within and between rows, as well as by assessments of damage to plants and yield.
To determine the density of wireworm larvae, traps were established in each plot and borders and were collected in 15 days and 30 days and were assessed separately. Traps were collected after 15 days, reestablished and again collected after 30 days. Whole set up was established once again and traps were again collected after 30 days. After collection traps were processed in berlese funnels and wireworms were separately collected from each plot. Later, they were identified using the keys of wireworm described by Etzler et. al (2013) and were used for lab study. Three wireworm species were identified from different fields which are, Limonius californicus, Hypnoides bicolor, and Aeolus mellilus.
Evaluating the effectiveness of trap crops (Exp 1)
Our previous results indicated that pea and lentil attracted significantly higher wireworms than wheat and other traps crops tested (canola, sweet corn, Montrail durum and Metealfa barley). Therefore, further experiments are conducted on the effect of seeding density on movement of wireworms on spring wheat. In 2017, experiment was established to study seeding density (Table 1). The experiment is designed in RCBD design for this study. All together there were 48 plots (4×3×4 = 48). An individual plot size was 3.6 m × 1.2 m. Buffer zone of 0.6 m was kept in between the plots. The cultivar of crop varieties used in 2017 are: Montech pea, Merrit lentil and Duclair wheat as control.
Evaluate the efficacy of entomopathogenic fungus (Exp 2a)
In the proposed study, different combinations of entomopathogenic fungi are tested for their efficacy against wireworms in spring wheat at two selected locations (Ledger and Valier) in Montana. The experiment is designed in RCBD design for this study. All together there were 48 plots (4×3×4 = 48). An individual plot size was 3.6 m × 1.2 m. Buffer zone of 0.6 m was kept in between the plots. Fungi are provided by USDA ARS (Sidney, MT) (Table 2).
Duclair seeds were seeded at the rate of 22 seeds/sq feet. Below mentioned fungi were applied in plots in furrows at two different rate 5 gms/plot and 10 gms/plot. The name used for fungus name and rate is mentioned in Table 2. Same names are used in result section and figures.
Evaluating the efficacy of fungi and reduced risk chemicals in lab bioassay (Exp 2b)
In the proposed study, different combinations of entomopathogenic fungi are tested for their efficacy against wireworms. In 2017, only a small setup is established to check the effect of entomopathogenic fungus in lab conditions. Other reduced risk chemicals will be tested and etomopathogenic fugus will be tried in 2018 (Table 3).
Rearing of wireworms and bionomics of wireworms (Exp 3)
To fulfill our objective 1 (lab bioassay of entomopathogenic fungus and reduced risk chemicals), and objective 3 (lab bioassay of entomopathogenic nematodes), we will require the continuous supply of wireworms. To fulfill this requirement, we plan to build a rearing bed for wireworms and rear them in field conditions. Both field and lab rearing techniques of wireworm have been mentioned by Furlan (1996 and 1998), nevertheless the field conditions will be different in Montana to rear these insects. A study of rearing of wireworms in Montana climate will also provide the opportunity to evaluate the overwintering behavior of wireworms and also their movement in the soil with changing weather conditions of Montana. Ovipositional behavior, effect of temperature and moisture and life cycle variation of selected species of wireworms will be also be studied simultaneously.
Statistical analysis
Analysis of variance (ANOVA) was used to analyze data in Excel. For both field trials, we used date of sampling as a blocking factor and performed ANOVA. Paired T-test was also applied to compare within group and P-values < 0.05 were considered significant.
Results
Wireworm species composition
The identification of wireworm species composition was performed in 2017 at both research locations- Valier and Ledger. Overall, three wireworm species- Limonius californicus, Hypnoidius bicolor and Aeolus mellilus were observed regardless of study locations.
However, in both locations, A. mellilus was the most predominant species followed by H. bicolor and L. californicus and A. mellilus at both sampling times.
Evaluating the effectiveness of trap crops
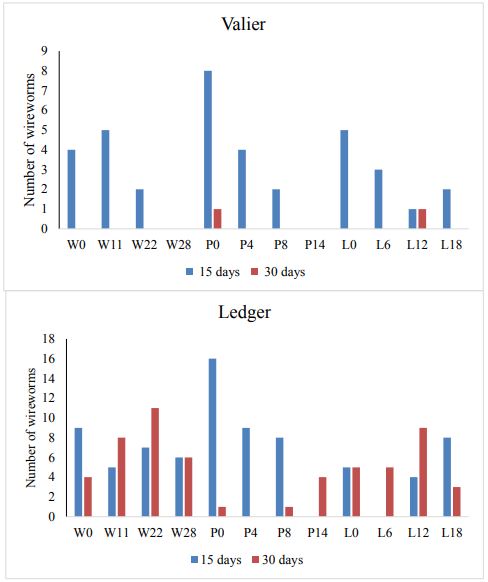
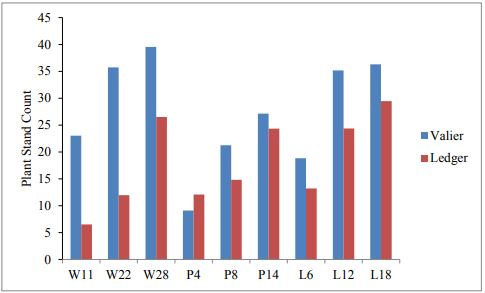
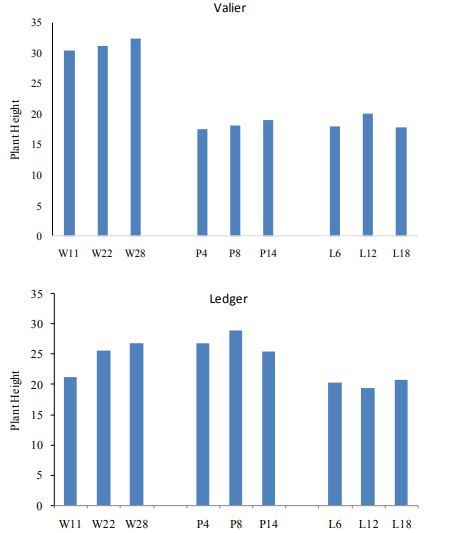
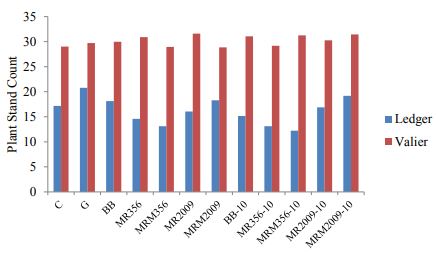
In 2017, at both locations plant counts, number of wireworms and yield is compared. In comparison to Valier site, Ledger site had greater wireworm pressure (Figure. 2). Hence variation in plant count is noticed and significant difference is found when t-test is applied (P<0.01; df=1, F=8, Figure 3). Nevertheless, no significance difference is found between the different seed rates of wheat, pea and lentil in terms of both plant count and plant height.
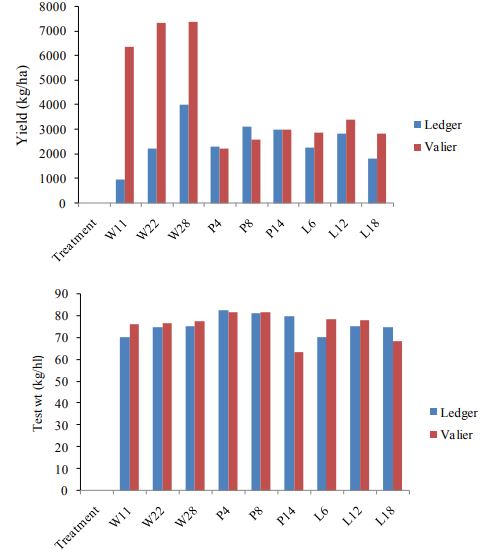
Both pea and lentil performed significantly well at the rate of P8 and L12 respectively (Figure 3,4). Same rate is observed to attract more wireworm and therefore higher spring wheat yield is found to be associated at this rate. Difference in number of wireworms collected after 15 days and 30 days is also observed, although significant only associated with P4 and P8 at Valier. Significant variation in spring wheat yield is found when compared between two sites (P<0.01; df=2; F=4.9; Figure 5).
Evaluate the efficacy of entomopathogenic fungus
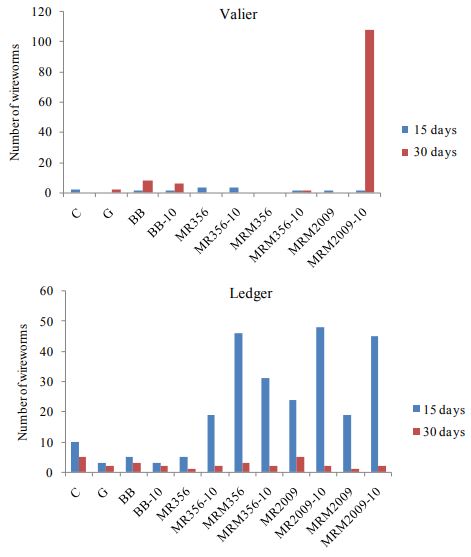
In 2017, at both locations plant counts, number of wireworms associated, and yield of spring wheat treated with entomopathogenic fungus at two rates is compared. In comparison to Valier site, Ledger site had greater wireworm pressure (Figure. 6). Greater association of wireworms is observed with MRM356, MR2009 (10gms) and MRM2009 (10 gms). At Ledger site number of wireworms collected after 15 days were significantly higher that
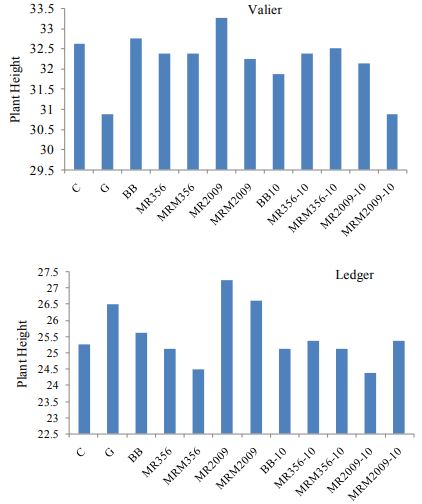
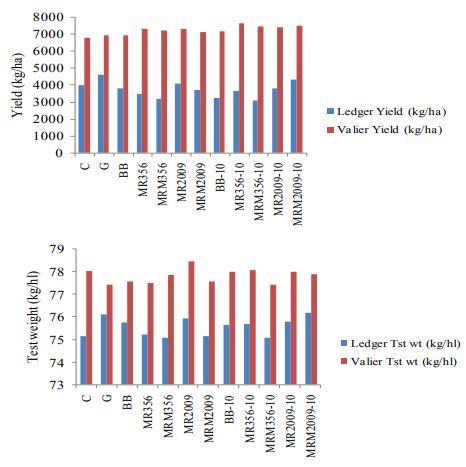
number of wireworms collected after 30 days (P<0.01; df=21, F=3.59). Nevertheless plant count and plant height is not found to be significantly different at both sites (Figure. 7, 8). Yield and test weight both are significantly different at both sites (P<0.01; df=11; F=5).
Greater yield is associated with MR356 and MRM2009. Similarly greater test weight is associated with MR2009 (Figure 9).
Evaluating the efficacy of fungi and reduced risk chemicals in lab bioassay:
With limited number of wireworms small bioassay was setup to test the efficacy of entomopathogenic fungus against wireworms. This study was only conducted over 13 days and showed best results with MR356 and MRM2009 (Figure 10).
Rearing of wireworms and bionomics of wireworms
As per discussed design (Furlan 1996 and 1998) the wireworm rearing cage is under construction and the proposed size is 1 m x 1 m x 2 m deep. We consider that 2 m depth will provide the wireworms to move during peak winters in Montana. Iron boxes, closed at the bottom by a plastic net (0.5mm mesh), will be placed in the ground and filled with air dried soil. The cages will be closed at the top by a net (2mm mesh) that will allow the rain to penetrate but not alter the average soil temperature.
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank summer intern Gaby Drishinski for assistance with field work.
References
Adhikari, A. and G.V.P. Reddy. 2017. Evaluation of trap crops for the management of wireworms in spring wheat in Montana. Arthropod Plant Interact. 11: 755–766. DOI 10.1007/s11829-017-9533-5.
Reddy, G.V.P., K. Tangtrakulwanich, S. Wu, J.H. Miller, V.L. Ophus, J. Prewett, and S.T. Jaronski. 2014. Evaluation of the effectiveness of the entomopathogens for the management of wireworms (Coleoptera: Elateridae) on spring wheat. J. Invertebr. Pathol. 120: 43–49.
Etzler, F.E. 2013. Identification of economic wireworms using traditional and molecular methods. M.S. thesis dissertation, Montana State University, Bozeman, Montana
Furlan, L. 1998. The biology of Agriotes ustulatus Schäller(Col., Elateridae). II. Larval development, pupation,whole cycle description and practical implications. J. Appl. Entomol. 122: 71–78.
Furlan, L. 1996. The biology of Agriotes ustulatus Schäller (Col., Elateridae). I. Adults and oviposition. J. Appl. Entomol. 120: 269–274.
White, G.F. 1927. A method for obtaining infective nematode larvae from cultures. Science 66:302–303.
Table 1: Seeding density of the trap crops.
|
Treatment |
Crop |
Seeds/Sq.ft. |
|
T1 |
Wheat |
0 |
|
T2 |
|
11 |
|
T3 |
|
22 |
|
T4 |
|
28 |
|
T5 |
Pea |
0 |
|
T6 |
|
4 |
|
T7 |
|
8 |
|
T8 |
|
14 |
|
T9 |
Lentil |
0 |
|
T10 |
|
6 |
|
T11 |
|
12 |
|
T12 |
|
18 |
Table 2: Material, rate, and method of application in each treatment in field.
|
Treatment |
Material |
Rate |
Source |
|
T1: |
Control (Water) |
|
|
|
T2: |
Gaucho® (Imidacloprid) |
0.157 ml/L (2.4 oz/45.352 kg/seed) |
Bayer Crop Science |
|
T3: |
Beauveria bassiana GHA Granules (BB) |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T4: |
Metarhizium robertsii DWR356 Granules (MR356) |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T5: |
Metarhizium robertsii DWR356 on millet substrate (MRM356) |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T6: |
Metarhizium robertsii DWR2009 Granules (MR2009) |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T7: |
Metarhizium robertsiiDWR2009 on millet substrate (MRM2009) |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T8: |
Beauveria bassiana GHA Granules (BB-10) |
(20lb/acre) 10 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T9: |
Metarhizium robertsii DWR356 Granules (MR356-10) |
(20lb/acre) 10 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T10: |
Metarhizium robertsii DWR356 on millet substrate (MRM356-10) |
(20lb/acre) 10 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T11: |
Metarhizium robertsii DWR2009 Granules (MR2009-10) |
(20lb/acre) 10 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T12: |
Metarhizium robertsiiDWR2009 on millet substrate (MRM2009-10) |
(20lb/acre) 10 gms/plot |
Stefan T. Jaronski USDA ARS |
Table 3: Treatments for lab bioassay
|
Treatment |
Material |
Rate |
Source |
|
T1: Control |
Water |
|
|
|
T2: Beauveria bassiana GHA Granules |
Beauveria bassiana GHA Granules |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T3: Metarhizium robertsii DWR356 Granules |
Metarhizium robertsii DWR356 Granules |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T4: Metarhizium robertsii DWR356 on millet substrate |
Metarhizium robertsii DWR356 on millet substrate |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T5: Metarhizium robertsii DWR2009 Granules |
Metarhizium robertsii DWR2009 Granules |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T6: Metarhizium robertsii DWR2009 on millet substrate |
Metarhizium robertsii DWR2009 on millet substrate |
(10lb/acre) 5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T7: Entrust® |
spinosad |
0.69g/1000ft2 (1 oz/acre) (0.091 ml/L of water) |
Dow Agro Science LLC, Indianapolis, IN |
|
T8: Aza-Direct® |
Neem |
25 g/L |
Gowan Company |
|
T9: Pyganic®1.4 EC |
pyrethrum |
16 fl. Oz./acre |
McLaughlin Gormley King Company (Minneapolis, MN)?Valent BioSciences |
|
T10: Met52® |
Met52 Microsclerotial granules (M. brunneum) |
20 lb./acre. |
Marrone Bio Innovations, Davis, CA |
|
T11: Met52® |
Met52 Corn grit granules (M. brunneum) |
20 lb./acre. |
Marrone Bio Innovations, Davis, CA |
|
T12: Xpectro® OD |
pyrethrin + B. bassiana GHA |
2.5 ml/L of water |
LAM International (Butte MT) |
Table 4: Treatments for entomopathogenic nematodes.
|
Entomopathogenic nematode species |
Strain |
Source |
|
Steinernemacarpocapsae |
All strain |
David Shapiro(USDA ARS) |
|
Cxrd strain |
David Shapiro(USDA ARS) |
|
|
Steinernemafeltiae |
SN strain |
David Shapiro(USDA ARS) |
|
Heterorhabditis bacteriophora |
HP88 strain |
David Shapiro(USDA ARS) |
|
VS strain |
David Shapiro(USDA ARS) |
|
|
Steinernema riobrave |
355 strain |
David Shapiro(USDA ARS) |
|
7-12 strain |
David Shapiro(USDA ARS) |
|
|
Heterorhabditisfloridensis |
K22 strain |
David Shapiro(USDA ARS) |
|
Heterorhabditis georgiana |
Kesha strain |
David Shapiro(USDA ARS) |
|
Steinernemararum |
17C&E |
David Shapiro(USDA ARS) |
Figure. 2: Number of wireworms collected at Ledger and Valier in 2017.
Figure 3: Mean number of plant stand count at both locations.
Figure 4. Plant height of spring wheat, pea and lentil at Ledger and Valier in 2017.
Figure 5. Yield (kg/ha) and test weight (kg./hl) of spring wheat, pea and lentil at Ledger and Valier in 2017.
Figure 6. Number of wireworms after 15 days and 30 days at Valier and Ledger in various plots treated with entomopathogenic fungus.
Figure 7. Plant stand count at Valier and Ledger in various plots treated with entomopathogenic fungus.
Figure 8. Plant height of spring wheat plants at Valier and Ledger treated with entomopathogenic fungus.
Figure 9. Yield (kg/ha) and test weight (kg/hl) of spring wheat at Ledger and Valier treated with entomopathogenic fungus.
Fig. 10. Growth of Metarhizium robertsii DWR2009 (millet substrate) on Hypnoidus bicolor dead larvae after 9 days(A) and 13 days (B).