Field efficacy of Bacillus thuringiensis galleriae strain SDS-502 for the management of alfalfa weevil and the impact on Bathyplectes spp. parasitization rate
Principal Investigators: Gadi VP Reddy1 and Stefan Jaronski2
Project Personnel: Govinda Shrestha1
1Montana State University, Western Triangle Agricultural Research Center, Conrad MT, 59425
2USDA-ARS-PARL, 1500 N. Central Ave. Sidney, MT 59270
Aim of the Study
One of our goals was to determine the efficacy of a commercial formulation of SDS-502 against the Hypera postica under Montana conditions. Observations by Rand (2013) suggested that efficiency of Bathyplectes curculionis and Oomyzus incertus, the predominant parasitoids in Montana alfalfa, was somewhat inversely proportional to host numbers, parasitism being density independent. We, therefore, also wanted to determine if a partial reduction of larval H. postica populations with a Bacillus thuringiensis would yield to greater parasitoid efficiency, manifested as a greater percent parasitism among the surviving larvae. If there would be complementarity between the microbial and the parasitoids, overall H. postica population suppression would be greater.
Alfalfa weevil Larvae feeding on alfalfa leaves
Alfalfa weevil larval parasitoids
Materials and Methods
Locations of alfalfa field trials
The research reported here was conducted in 2016. The experiments were conducted at two locations: Valier (N 48o 35.192 W112o 21.169) and Conrad (N 48o 30.206 W112 o14.350), Pondera County, in the Gold Triangle region of Montana, USA. Both alfalfa fields had reached economic threshold level (1 larvae/stem), as determined by larval numbers prior to field selections. Ages of the crop ranged from 3 to 5 years and the area of the Valier and Conrad fields were 68 and 16 ha, respectively. Alfalfa was grown according to recommended industry standards and both were irrigated fields.
A randomized complete block design (RCBD), with four replicates per treatment, was used. Each treatment plot was 6 × 6 m, and separated from each other by 3 m buffer zones to avoid any overlap of treatment effects. Plots were situated at least 6 m inside from field edge.
Bacillus thuringiensis galleriae SDS-502 application
A commercial formulation of B. thuringiensis galleriae STS-502 (BeetleGone®) (76.5% a.i.,
>0.85x1010 CFU/g) was provided by Phyllom BioProducts Corporation, Oakland, California, USA. The low and high recommended application rates of BeetleGone corresponding to 2.2 and
4.4 kg, respectively, per hectare in 234 L ha-1 were used for experiments. NuFilm® 17 (Miller Chemical and Fertilizer, LLC) was added to each BeetleGone treatment (583 ml ha-1) as a sticker. The spray suspension was prepared by mixing the product materials with water, followed by addition of the NuFilm17, and agitated well before spray application. The diluted NuFilm 17 served as a carrier control treatment.
Treatments were applied using a CO2- pressurized backpack sprayer calibrated to deliver 252 L ha-1 through a two-person, 3.66 m, boom with TeeJet nozzles spaced 0.46 m apart. Each plot was sprayed in two swaths. The spraying activity was performed between 6-8 am.
Sampling
Alfalfa weevil larvae population
Hypera postica larvae were sampled in all plots to determine the treatment effects. Sampling was conducted 2 days before treatment application, and 3 and 7 days after applications. Ten samples were taken from each treatment plot, with 3 alfalfa stems/sample, and the sampling was performed along an N-shaped transect beginning 1 m into the plot. Alfalfa stems were detached from the base of plants with help of scissors, placed into one zipper-lock bag, and kept in a picnic cooler. The samples were returned immediately to the lab and larvae dislodged from foliage by vigorous shaking in a plastic bucket. The larvae were categorized into two age classes-‘young’ (L1-L2) and ‘old’ (L3-L4).
Parasitization rate of Bathyplectes spp.
Parasitism by Bathyplectes spp. in H. postica larvae was assessed in treatment plots with larvae collected at 7 days post application. Both stem-cut and sweep net sampling were used.
Sweeping was conducted with a standard sweep net (180o arc) with 20 sweeps in each treatment plot.
The larvae collected from each treatment plot were kept in plastic zipper-lock bags with some alfalfa foliage and transported immediately to laboratory. In the lab, H. postica larvae from each treatment plot were transferred into a large paper bag with a paper towel in the bottom. Fresh alfalfa foliage (1-2 healthy stems) was placed in each bag, and the top of the bag was folded multiple times and secured with a large binder clip. Fresh foliage was added every other day as needed and dried out foliage was left in a bag in order to avoid risk of losing insects. All bags were kept at room/lab temperature for 14 days at which time most insects had pupated or eclosed into adults. Hypera postica larvae parasitized by Bathyplectes spp. were determined by presence of dark brown, football-shaped cocoons with un-raised white band around the cocoon or with white equatorial band that is not raised (Tharp, 2015).
Data analysis
The data were analyzed with R 2.15.1 (R Development Core Team, 2011). For all data, a test with a normal quantile-quantile plot was performed to confirm normality of the data and equality of variance. Where appropriate, Tukey`s contrast pairwise multiple comparisons were used to test for significant differences in means (Hothorn et al., 2008). Furthermore, the data were subjected to angular transformation prior to statistical analysis.
Alfalfa weevil population
The percentage reduction of alfalfa weevil population was calculated relative to the initial larval population (assessed 2 days before spraying) as follows:
Alfalfa weevil density reduction (AWDR) (%)
= 𝐴𝑊𝐷𝑅𝑠𝑡0−𝐴𝑊𝐷𝑅𝑠𝑡1 × 100;
𝐴𝑊𝐷𝑅𝑠𝑡0
Where AWDRst0 represents the number of H. postica larvae recorded at each treatment plot before the BeetleGone application and AWDRst1 is the number of H. postica larvae recorded at each treatment plot in each sampling time (3 days or 7 days after BeetleGone or carrier control treatments) (Shrestha et al., 2015).
The overall data were fitted to a linear mixed model with sampling time interval, SDS-502 rate and H. postica populations per replicate as fixed effects (categorical variables converted to factors), the variation in H. postica populations (1|Unit) as random effect and the mean H. postica populations per treatment as response variable using the function “lmer”. The mean alfalfa weevil population per treatment was calculated using the “Summaryby” work package (doBy). The model was then simplified with stepwise removal of factors having no effect. The Kenward-Roger test was run using the function “KRmodcomp” to compare the models (Halekoh and Højsgaard, 2012). One-Way Analysis of Variance (ANOVA) was carried out to determine the effect on H. postica population across treatment levels at each sampling time.
H. postica percent control level due to SDS-502 rates was further calculated by using the formula given by Henderson and Tilton (1955):
= 100 [1 − 𝑇𝑎×𝐶𝑏 ]
𝑇𝑏 ×𝐶𝑎
Here Tb is the number of H. postica larvae collected per sampling unit before treatment, Ta the number collected after treatment, Cb the number collected from the carrier control plot before treatment, and Ca the number collected from the carrier control plot after treatment of test plots.
Parasitism
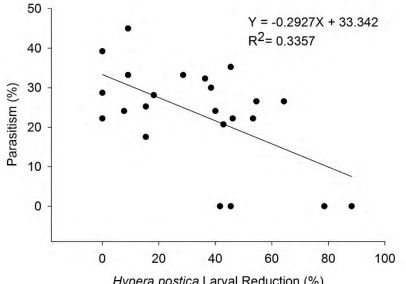
One Way-ANOVA was performed to evaluate whether the spray of SDS-502 had an effect on parasitism levels by Bathyplectes spp. The parasitism percentage was calculated as numbers of parasitoid pupae formed / total number of H. postica larvae reared from each plot × 100. Linear regression was further used to analyze the correlation between mean parasitism percentage and mean 7 days weevil larvae reduction percentage after the treatment application through stem- cut method. Data from Valier and Conrad locations were pooled for analysis.
Results
Effects on alfalfa weevil populations
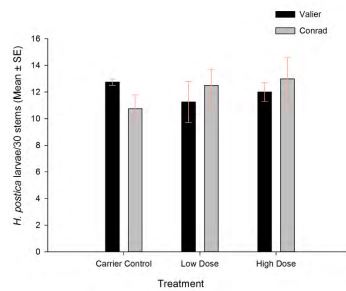
The overall mean number (± SE) of H. postica larvae per 30 stems 2 days before the treatment applications at the Valier and Conrad locations ranged from 11.25 to 12.75 and 10.75 to 13.00, respectively, across all plots (Fig.1). Mean number of post-treatment H. postica larvae per 30 stems (± SE) for Valier location varied from 8.00 ± 0.40 to 13.00 ± 1.58 and 5.25 ± 0.85 to
11.75 ± 0.85 respectively, at the 3 days and 7 days post-applications. The corresponding values for Conrad location were 7.75 ± 0.25 to 11.75 ± 0.85 and 4.75 ± 0.95 to 9.50 ± 0.50 respectively. There were significant treatment effects for both SDS-502 rates (Valier: F = 13.19; df = 2, 18; P < 0.0001; Conrad: F = 15.20; df = 2, 18; P < 0.0001) and at both sampling times (Valier: F = 6.09; df = 1, 18; P < 0.05; Conrad: F = 5.66; df = 1, 18; P < 0.05). There were no interaction effects between treatments and sampling times (Valier: F = 0.10; df = 2, 18; P > 0.05; Conrad: F = 0. 06; df = 2, 18; P > 0.05).
Across the treatment levels, the significant difference in H. postica numbers occurred at 3 days post treatment (Valier: F = 5.32; df = 2, 9; P <0.05; Conrad: F = 6.78; df = 2, 9; P <0.05) and 7
days (Valier: F = 8.93; df = 2, 9; P <0.01; Conrad: F = 9.98; df = 2, 9; P <0.01). The percentage reduction of H. postica larval populations with the two SDS-502 treatments was rate-dependent. (Table 1). The SDS-502 provided 27-40% reduction in weevil numbers at the low label rate and 55-59 % for the high label rate (Table 1).
Based on the Henderson and Tilton correction (1955), mean alfalfa weevil control level ranged 12-32% for the low rate and 36-51% for the high rate of SDS-502 at the 3 days and 7 days post- applications at the Valier location. Similarly, at Conrad location, average weevil control level for low and high rate of SDS-502 varied from 25-40 % and 38-54% respectively at the 3 days or 7 days after treatments.
Parasitism level
Bathyplectes spp. were recorded in both alfalfa fields. The mean parasitism levels varied from 5-26% and 17-36% respectively at Valier and Conrad research sites (Table 2) There were no significant differences in rates of parasitism among treatments at both Conrad (stem cut: F =
3.02; df = 2, 9; P = 0.09 and sweep netting: F = 0.87; df = 2, 9; P = 0.45) and Valier (sweep net: F = 2.20; df = 2, 9; P = 0.17) with one exception. At Valier, in the stem-cut samples from high rate of SDS-502 plots only, there was a significantly lower mean parasitism rate (5.0% ± 5.00) compared to all other treatments (Table 2). No significant relationship between parasitism levels and H. postica reduction percentage at the 7 days post-application was recorded based on R2 value (0.306) as predicted by a linear regression equation (Fig. 2).
Acknowledgements
We would like to thank Connie Miller, Montana State University, and Rob Schlothauer, USDA ARS, for assistance with field work. The authors acknowledge Montana Agricultural Experiment Station for funding this research, Grant Accession # 232056.
References
Halekoh, U., Højsgaard, S., 2012. pbkrtest: parametric bootstrap and Kenward Roger based methods for mixed model comparison, 2012. R package version 0.3-4.
Henderson, C.F., Tilton, E.W., 1955. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 48, 157-61.
Hothorn, T., Bretz, F., Westfall, P., 2008. Simultaneous inference in general parametric models.
Biom. J. 50, 346-363.
R Development Core Team, 2011. R: A Language and Environment for Statistical Computing.
R Foundation for Statistical Computing, Vienna.
Rand, T. A., 2013. Host density drives spatial variation in parasitism of the alfalfa weevil,
Hypera postica, across dryland and irrigated alfalfa cropping systems. Environ. Entomol. 42, 116-122.
Table 1. Cumulative percentage reduction (mean ± SE) of Hypera postica larval population on alfalfa plants after Bacillus thuringiensis SDS-502 or carrier control application.
|
Location |
Sampling time |
|
Treatment |
|
|
|
|
Carrier Control |
Low Dose |
High Dose |
|
Valier |
3 DAT |
1.9 ± 1.92b |
11.0 ± 6.88b (12.5 ± 7.65) |
33.2 ± 2.07a (35.7 ± 1.99) |
|
|
7 DAT |
9.2 ± 3.68b |
26.8 ± 10.90b (31.9 ± 6.96) |
55.1 ± 8.29a (51.0 ± 8.99) |
|
Conrad |
3 DAT |
5.8 ± 5.77b |
24.9 ± 5.07b (31.3 ± 4.64) |
38.3 ± 5.96a (43.5 ± 5.44) |
|
|
7 DAT |
14.2 ± 8.37b |
39.6 ± 5.17a (31.6 ± 5.88) |
59.5 ± 10.90a (54.2 ± 12.34) |
Different letters within a row indicate significant differences between treatments (Tukey test, p< 0.05). The values in parentheses denote the mean H. postica control levels calculated based on Henderson and Tilton correction (Henderson and Tilton, 1955).
Table 2. Mean percent parasitism (± SEM) by Bathyplectes spp. in Hypera postica larvae 7 days after Bacillus thuringiensis SDS-502 or carrier control application.
|
Location |
Sampling Method |
|
Treatments |
|
|
|
|
Carrier Control |
Low Dose |
High Dose |
|
Valier |
Stem cut |
19.3 ± 1.68a |
15.8 ± 2.17a |
5.0 ± 5. 00b |
|
|
Sweep net |
25.8 ± 3.05a |
18. 2 ± 2.41a |
24.1 ± 2.84a |
|
Conrad |
Stem cut |
36.3 ± 5.54a |
22.4 ±4.03a |
17.5 ± 6.85a |
|
|
Sweep net |
26.8 ± 5.28a |
20.2 ± 2.53a |
21.3 ± 3.62a |
Different letters within a row indicate significant differences between treatments (Tukey test, p< 0.05).
Figure 1. Average numbers (± SE) of Hypera postica larvae recorded 2 days before treatments at Valier and Conrad, Montana
Figure 2. Relationship between Bathyplectes spp. parasitism and Hypera postica larval reduction levels, 7 days post-application. Valier and Conrad locations data were pooled for analysis.