Efficacy of entomopathogenic nematodes (EPN's) against wireworms and non-target organisms (Green lacewings and Ladybugs)
Principal Investigator: Gadi V.P. Reddy
Project Personnel: Ramandeep Kaur Sandhi, Shabeg S. Briar, Anamika Sharma, Ramadevi Gadi and Deb Miller
Montana State University, Western Triangle Agricultural Research Center, Conrad MT, 59425
Aim of the Study
The aim of the study was to investigate whether Entomopathogenic (insect pathogenic) nematodes are able to kill wireworms and have any negative effect on non-target organisms such as green lacewings, Chrysoperla carnea (Neuroptera: Chrysopidae) and lady bugs, Adalia bipunctata (Coleoptera: Coccinellidae).
Materials and Methods Wireworms
Wireworm collection
The larvae of different instars of wireworms were collected from two field locations (Conrad and Pendroy were collected by using stocking traps. The stocking traps with soaked wheat seeds were put at different spots beneath the soil in the wireworm infested fields and covered with the plastic sheets. After 15-20 days, the stocking traps were collected and brought back to the laboratoryand, replaced with new ones every time. The stocking traps with wireworms were put in the Berlese Funnels for 12 hours and wireworms were collected underneath in a glass jar. In addition wireworms were also manually handpicked from the soil samples collected from the infested fields (Figure 1). The collected wireworms were categorized into small, medium and large based on their body size. Mainly three types of wireworm species viz. Limonius californicus, Hypnoides bicolor, and Aeolus mellillus considered for collection of larvae in 2017.
Wireworms collected and separated from the plant roots or soil samples were stored in an incubator at 8° C in plastic cups with sterilized soil containing wheat seeds added as their food.
Figure 1. Wireworm collection from the soil and plant roots (Left) and separation from the soil samples using Berlese Funnels in the lab (Right).
EPN’s species
Ten nematode strains were obtained from Dr. David Shapiro, USDA ARS, Byron, GA (Table-1).
|
S. No |
Entomopathogenic nematode species |
Strain |
|
1 |
Steinernema carpocapsae |
A11 strain |
|
2 |
Cxrd strain |
|
|
3 |
Steinernema feltiae |
SN strain |
|
4 |
Heterorhabditis bacteriophora |
HP88 strain |
|
5 |
VS strain |
|
|
6 |
Steinernema riobrave |
355 strain |
|
7 |
7-12 strain |
|
|
8 |
Heterorhabditis floridensis |
K22 strain |
|
9 |
Heterorhabditis georgiana |
Kesha strain |
|
10 |
Steinernema rarum |
17 c+e strain |
Table 1: Different entomopathogenic nematode species used in the present study Entomopathogenic nematodes rearing
The EPNs obtained were further reared on waxworm, Galleria mellonella (Lepidoptera: Pyralidae) larvae. The five later-instar larvae were placed on the filter paper in the petri dishes. 500-1000 infective juveniles (IJs/ml) were inoculated in each petri dish with approximately 100-
200 IJs for single larva in the petri dish. Once inoculated, petri dishes were left at room temperature (20-25° C) for EPNs to enter the larvae and cause infection. After 3-5 days, EPNs infected larvae were placed on the white traps for rearing (Figure 2). In about 7 to 10 days later, EPNs were washed from the white traps with distilled water using pipette into the flasks.
Figure 2. Infected waxworm larvae on white trap EPNs concentrations
For preparing different concentrations, EPNS were collected in distilled water (100-1000 ml). After making the solution, 1 ml of the suspension was taken onto the counting slide and number of EPNs were counted under the stereo-microscope. This was repeated 10 times to express an average number of EPNs per ml of solution. First, the higher concentration was made (2000 IJ/ml) and on the basis of this concentration, further dilutions of 1000, 500, 250, 100, 50 IJ/ml were made. EPN concentrations were stored in the incubator at 8° C temperature.
Laboratory bioassay
In the bioassay, three species of wireworms (medium size larvae) were used and due to low number of wireworm’s availability, only selected EPN strains were tested in this study. Plastic cups of 30-ml volume were filled with 25 g of sandy loam sterilized soil and few soaked wheat seeds were provided as a food source for the larvae. Two wireworm larvae were placed in the cups. Different concentrations of EPNs (i.e. 50, 100, 250, 500, 1000 and 2000 IJ/ml) were inoculated onto the soil surface in 1 ml of water. Ambient moisture was maintained constantly for EPNs movement in the soil. In the control treatment, one ml of water was added. Cups were capped with 4-5 holes and then placed in the growth chamber at 25° C at 70-80 % RH in the dark (Figure 3). Larval mortality was observed at every 24 hrs intervals for up to 30 days. In case of L. californicus bioassay, all the EPN strains were used except S. riobrave 7-12 strain. In case of H. bicolor, seven EPN strains including S. carpocapsae (A11 and Cxrd strain), S. feltiae SN strain, H. bacteriophora HP88 strain, S. riobrave 355 strain, H. floridensis K22 strain and S. rarum 17C&E strain were used. On the other hand, five EPN strains; S. carpocapsae (A11 and Cxrd strain), S. feltiae SN strain, H. floridensis K22 strain, and S. rarum17C&E strain were used on A. mellilus. The dead larvae were dissected to check for the presence of EPNs under the stereomicroscope and confirm that mortality was due to the EPNs infection.
Figure 3: Inoculated cups in incubator
Beneficial insects
Two beneficial insects; Green lacewing, Chrysoperla carnea and two spotted lady beetle, Adalia bipuncatata were used in this laboratory bioassay. Second or third instar larvae of these insects were obtained from the Biobest Company.
EPNs concentrations: Only four EPN concentrations of 500, 250, 100, and 50 IJ/ml were used.
Laboratory bioassay
Five C. carnea larvae were placed in the 9 cm diameter petri plates with a piece of whatman filter paper and few eggs of Ephestia kuehniella (Lepidoptera: Pyralide) as a food source for the larvae. The EPN concentrations of 500, 250, 100 and 50 IJ/ml of water were inoculated individually to each petri plate (Figure 4). These petri dishes were kept in the growth chamber at 25° C at 70-80% RH and 16:8 photoperiod. The control received distilled water without EPNs. Each treatment was replicated five times. The larval mortality was observed at the 24 hr intervals for upto 7 days. The dead larvae were dissected under the stereomicroscope to confirm the EPNs infection. The same procedure was followed for the A. bipunctata larvae. In case of C. carnea, four EPN strains: S. feltiae SN strain, S. carpocapsae A11 strain, H. bacteriophora HP88 strain, and S. rarum 17C&E strain were used. On the other hand, for A. bipunctata, only three EPN strains, S. feltiae SN strain, S. carpocapsae A11 strain, and S. rarum 17 c+e strain were used.
Figure 4. Petri dishes with A. bipunctata larvae inoculated with nematodes
Results
Wireworms
The mean larval mortality were calculated on the basis of average of two replication. Since only two replications were possible in this study only mean values were calculated while this data set did not allow us to perform any parametric statistical analysis. However, more replications will be included in future studies in order to obtain enough data and perform the mean comparison tests among the treatments. The infected L. californicus and A. mellilus are shown in Figure 5.
Figure 5. Nematodes infected larvae; Left: L. californicus, Right: A. mellilus
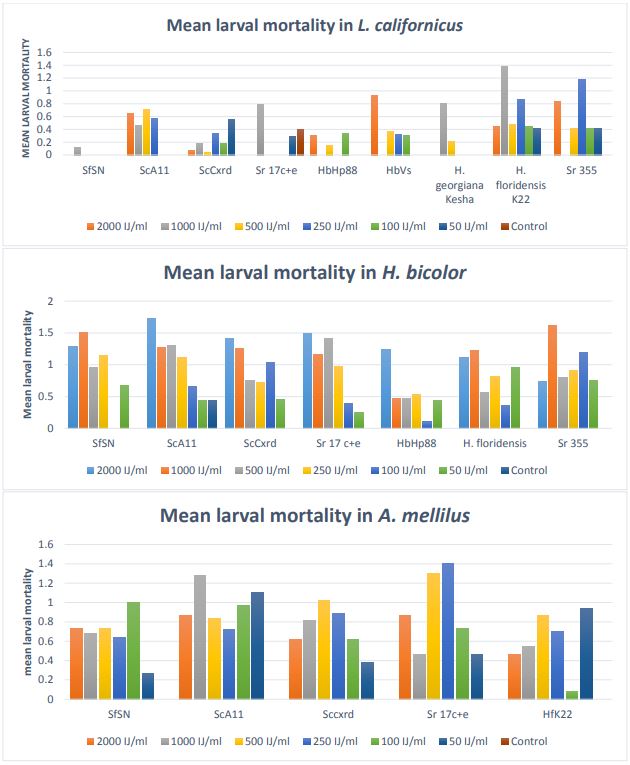
The mean larval mortality in L. californicus, H. bicolor, and A. mellilus is presented in Figure 6. In L. californicus, EPN strain H. floridensis showed high mortality (30-65%) followed by S. riobrave 355 (30-60%). The other strains also showed mortality but not comparable to H. floridensis K22 strain and S. riobrave 355 strain.
In H. bicolor, out of 7 strains evaluated, high mortality (20-80 %) was observed in S. carpocapsae A11 strain followed by S. feltiae SN strain, S. rarum 17 c+e, and S. carpocapsae Cxrd strain with mortality ranging between 25-70 %. S. riobrave 355 strain also showed mortality in this case.
In A. mellilus, S. rarum 17 c+e and S. carpocapsae A11 strain showed more mortality ranging between 35-70 %. Other three strains; S. feltiae SN strain, S. carpocapsae cxrd strain, and H. floridensis K22 strain showed almost same results.
Conclusions
Overall, different EPN strains caused high mortality in different wireworm species. H. floridensis K22 strain, S. carpocapsae A11 strain, S. rarum 17 c+2 strain were found to be more infective than other strains. These results are based on the preliminary studies as number of larvae used in the bioassay were low. In the coming years, more research will be done with additional number of larvae and bioassays will be repeated with all the 10 EPN strains. Different size larvae (small, medium and large) will be used to check the efficacy of EPNs at different stages of the life cycle of wireworms. The time to cause mortality in different EPN strains will be observed carefully. Field/greenhouse experiments will also be conducted to check their effects in fields.
Beneficial insects
The mean larval mortality for seven days was calculated based on the five replications. In most of cases, higher the EPN concentration, higher the mortality. Mortality observed in the control was not due to nematodes but because of unfavorable environmental conditions.
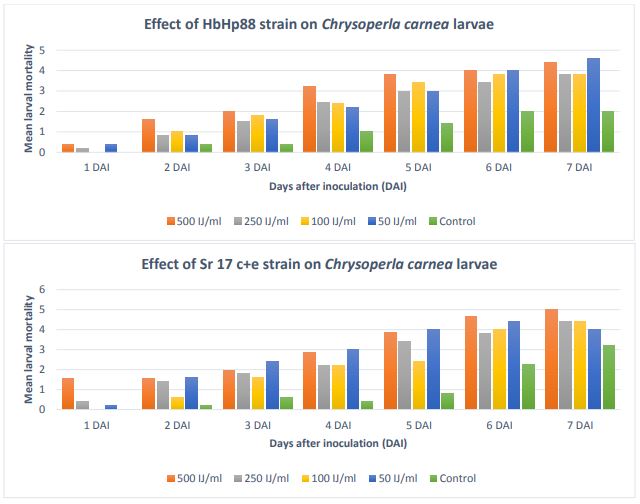
Figure 6. Mean number of larval mortality in L. californicus, H. bicolor, A. mellilus Chrysoperla carnea
Chrysoperla carnea
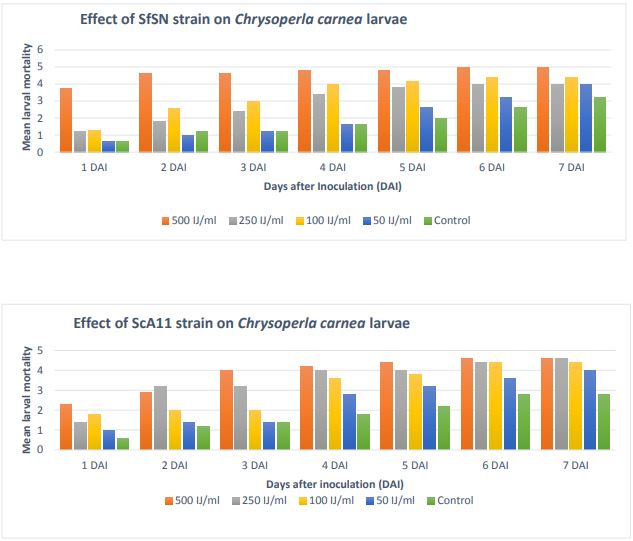
The mean larval mortality is shown in Figure 7. In S. feltiae SN strain, mortality
at very first day at 500 IJ/ml was approximately 75 %. Likewise, in the other three
concentrations, the mortality was about 50%. It went on increasing with the number
of days. On sixth day, it showed 100% mortality at 500 IJ/ml EPN concentration. In
the other concentrations also, mortality was around 70 to 80 %.
Figure 7. Mean number of larval mortality in Chrysoperla carnea
Similar trend was seen in S. carpocapsae A11 strain, as mortality was increasing with
the passing days. It was 40-50 % on day second and reached 90% at the end of week.
Although, in H. bacteriophora Hp88 strain, mortality was higher with the passing days,
unlike SfSN strain and ScA11 strain, the mortality during first 2 days was not high
reached 30% on day third and mortality was 80-90 % on day six. In S. rarum 17c+e strain,
the trend in the mortality was similar to HbHp88 strain except the mortality reached
100% on day seven at 500 IJ /ml EPN concentration. Adalia bipunctata
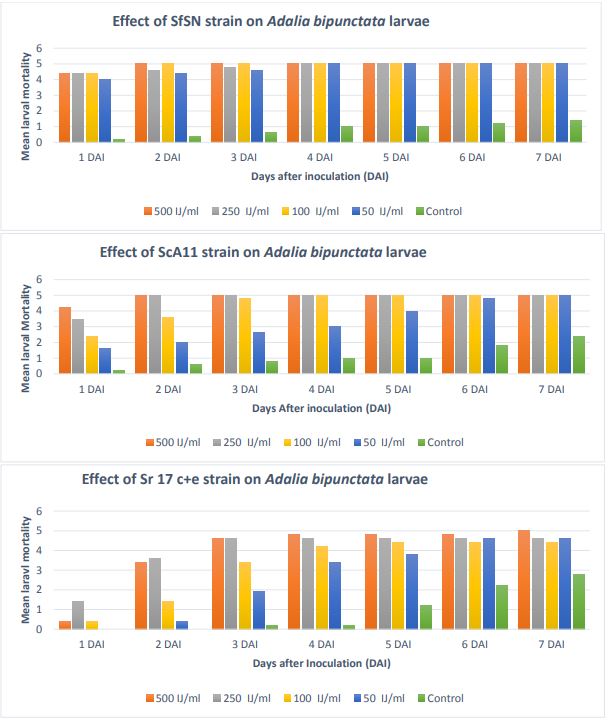
The mean larval mortality in A. bipunctata with S. feltiae SN strain, S. carpocapsae
A11 strain, and S. rarum 17 c+e is presented in Figure 8. In S. feltiae SN strain,
mortality was 85% on the very first day. It increased on day second and reached 100%
at 500 IJ and 100 IJ/ml concentrations. The mortality was 100 % at day fourth in all
the four concentrations. In S. carpocapsae A11 strain, higher the EPN concentration,
higher the larval mortality. On the second day, mortality was 100% in case of 500
IJ/ml and 250 IJ/ml concentrations. On day fourth, mortality reached 100% in case
of 100 IJ/ml EPN concentration. At the end of one week, 100% mortality was there in
all the four concentrations. In S. rarum 17 c+e, mortality was very low on day first.
It increased and reached 90-95 % on day fourth. However, 100 % mortality was observed
only at 500 IJ/ml on day seventh. In the other three concentrations, mortality was
around 90-95%.
Conclusions
Overall, in the tests with beneficial insects, the EPNs showed very high mortality in both the insects. Further research will be done with the other EPN strains. The bioassay will be repeated and other factors such as the surviving larvae are going through their developmental stages or not will be determined.
Figure 8. Mean larval mortality in Adalia bipunctata
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank, Gabby Drishinski and Carley Taft for assistance with the wireworm collection and laboratory work.