Managing wheat stem sawfly using synthetic plant defense elicitor chemicals
Principal Investigator: Gadi V.P. Reddy
Project Personnel: Govinda Shrestha, Shabeg Briar, John H. Miller, Julie Prewitt, Ramadevi L. Gadi and Debra Miller
Western Triangle Agricultural Research Center, Montana State University, 9546 Old Shelby Rd., P.O. Box 656, Conrad, MT 59425, USA
Aim of the Study
The aim of this study was: 1) to determine the effects of three synthetic plant defense elicitor chemicals (Acitgard, Cis-Jasmone and Neem) treatment application on wheat stem sawfly management.
Materials and Methods
Insect
Wheat stubbles containing wheat stem sawfly (WSS) diapausing larvae were collected from winter wheat fields in Pondera County, Montana from October 2016 to February 2017. Infested, ‘WSS-cut’ stems were stored in plastic deli round containers and maintained in a climate cabinet (5 ± 1ºC) for nearly three months in the dark to facilitate completion of obligatory larval diapause (Holmes, 1977). Afterwards, WSS infested stems were transferred in new deli containers. However, the half of these containers were first filled with garden soil, stem base placed beneath the soil and positioned in standing situation. About 50-60 stems were kept in each container, placed in insect cages (12 × 10 × 10 cm), and held at a laboratory temperature (19-21 ºC, 50–60% RH, 16:8 L: D) at Western Triangle Agricultural Research Center (WTARC), Montana State University, USA.
Containers were lightly moistened twice weekly with tap water to minimize the desiccation of the larvae. The insect cages were opened daily, and the emerged WSS adults were held in new insect cages with cotton pad pieces moistened with a solution of 10 % of honey in water until they were used for experiments. . In each insect cage, about 100 WSS adults were placed with about female and male ratio of 60:40. To minimize the host deprivation time, the laboratory bioassay experiments were performed with WSS female adults within 48 h of emergence. In our rearing system, adults emerged in three-four weeks from WSS infested stems.
Plants
Winter wheat variety ‘Yellowstone’ was selected as a source of plant material for both the lab and the field experiments. This variety is one of the most common winter wheat variety in Montana because of high yielding, excellent qualities (baking and noodle), and moderately resistance to plant diseases (dwarf smut and stripe rust), but; very susceptible to WSS damage. Winter wheat plants require a period of exposure to cool temperatures to trigger its stem elongation and reproductive induction. For the laboratory bioassay experiments the winter wheat seedling nursery plot was established at WTARC. Winter wheat seeds were seeded at the rate of 194 live seeds per m2 in September 2016.The nutrient and irrigation management tactics were as per the normal standard grower’s agronomic practices.
When the vernalization completed naturally in the next year spring 2017, the seedlings (two unfolded leaves) were transplanted into tapered square pots (13 × 13 × 13.5 cm) a density of three plants per pot. Plants were maintained in a greenhouse at 18-20 0C, 50–60% RH and natural light conditions until used for experiments. Each pot contained 1.62 kg of prepared soil mixture. The soil mixture has an equal proportion of sand, vermiculite, and peat moss, and N, P and K fertilizer at 17.13, 11.49 and 8.10 g/100 kg soil mixture respectively. Plants were watered four to five times weekly, and fertigated with Peters General Purpose Fertilizer (J. R. Peters, Allentown, PA) at 100 ppm in aqueous solution at fortnight interval. Nutrient application initiated when the plants reached the third leaf stage (Zadoks et al. 1974). Plants used for the laboratory experiments were at a developmental stage of Zadoks 33 when 2–3 nodes are visible. This stage was considered for experiments since WSS female adults preferred this wheat plant stage for oviposition, as reported by Holmes and Peterson (1960).
Synthetic plant defense elicitor (SPDE) chemicals source and rates
Actigard 50WG® was obtained from Syngenta (Fargo, ND) as a water-dispersible granular formulation containing 50 % active ingredient. Cis-Jasmone was obtained from Sigma-Aldrich (St. Louis, MO) as a soluble liquid formulation with 85 % purity. Azadirachtin (extracts from neem) containing 1.2 % emulsifiable concentration was obtained from Gowan Company (Yuma, AZ). For both the laboratory and field experiments, the chemicals were applied at the concentrations of 0.75 ml/L (Actigard), 0.5 ml/L (Cis-Jasmone) and 2.88 ml/L (Azadirachtin), which correspond to the doses recommended by the manufacturers for agricultural practices.
Lab experiments
WSS adult settling preference experiments
The bioassay experiments were performed to determine the WSS female adults settling preference for wheat plants (control) or wheat plants treated with SPDE chemicals as aforementioned. For the application of SPDE chemicals, potted wheat plants established in the greenhouse were transported to a spraying room. Spray application was made to individual plants using a 750 ml hand-held sprayer, with a spray volume of 20 ml per plant. After spraying, plants were allowed to dry for 1 h, and then transported to the collapsible cages. Plants treated with tap water served as a control treatment.
Four wheat plants were placed inside a cage. Two of the plants were SPDE chemicals treated and two were control to facilitate choice in the experiment. Each group of plants were further placed separately inside a cage. Subsequently, 10 female adults (48 hr old) were released at the center of the cage and allowed to settle overnight. However, insects were released five days after the Actigard treatment application to allow to induce resistance against insect pests in plants.
Following day, the position of each WSS adult (either on the SPDE chemicals treated, the water treated plants or elsewhere) was recorded in the morning (9:00-10:00 am) and the afternoon (15:00-16:00 pm) local time.
A similar procedure was followed to determine the WSS female adults settling behavior under no-choice condition. Except, one plant treated with each SPDE chemical or water (control) was placed in an insect cage and the number of insect released per cage was seven. The cages were maintained in a room at 19-21 ºC, 50–60% RH, 16:8 L: D. Both choice and no-choice experiments have four replications (each insect cage = one replicate) and conducted on two times.
Field experiments
Locations of winter wheat field trials
The experiments were conducted at three locations: Knees (N 48°00'08.5 W 111°21'51.8), Conrad (N 48°18'29.0 W 111°55'23.1) and Choteau (N 47°59'36.0 W112°06'49.9), in the Golden Triangle area of Montana, USA. All experimental locations were known to have moderate to high WSS infestation levels for many years. The experimental plots were seeded between the first and second week of September 2016 with a seeding rate of 194 live seeds per m2. The seeds were planted in four rows, with 30 cm between rows. Glyphosate (Roundup Powermax®) was applied at the rate of 2.5 L/ ha (the active ingredient of 540 g/L of acid glyphosate) before to the seeding to control weed growth. Fertilizer N, P, and K ratio at 224.2, 0, and 22.4 kg/ha was broadcasted while planting, and an additional application of 12.3, 25.2, and 0 kg/ha of these three nutrients were placed through seed plot drill.
A randomized complete block design (RCBD) with four replicates per treatment was used. Plots were 3.6 × 1.2 m separated by 0.60 m buffer zones to avoid cross contamination of treatments.
Monitoring of WSS adults
WSS adults usually begin to deposit eggs in developing wheat stems when the stem elongation is initiated during the spring season. Estimating the ideal application time for synthetic pesticides or SPDE chemicals, could be one of the critical factors for WSS management. Currently, no degree-day model has been established for determining WSS adult emergence (Knodel et al.
2011). In this study, two methods were used: 1) dissection of WSS infested stubble for monitoring the immature stages of WSS (Nansen et al. 2005) and 2) sweep netting the winter wheat fields for monitoring the adult emergence (Knodel et al. 2010). Based on these two methods, experimental plots and their adjacent winter wheat fields were scouted at a weekly interval from last week of April to until mid-June, 2017.
Application of SPDE chemicals
The field experiments consisted of three treatment types: 1) application of SPDE chemicals before to WSS egg laying, 2) after egg laying, and 3) before and after egg laying inside wheat plants. The considerations for multiple treatment categories were to determine the most vulnerable insect stages and wheat plants response to the spraying timing of SPDE chemicals.
All SPDE chemicals were applied on the same date (treatment 1: May 29, 2017; and treatment 2 & 3: June 6, 2017) at Knees and Choteau field trial locations. However, for Conrad location, the spraying activity started ten days later because of the late emergence of WSS adults. Treatments were applied using a SOLO backpack sprayer (SOLO, Newport News, VA). The sprayer was calibrated to deliver ca. 150 L mixture/ha based on nozzle flow and walking speed. Plants treated with water served as control. At all field trail locations, SPDE chemicals were applied between 4-6 wheat nodules stages.
Collection of wheat stems
Wheat stems were sampled in all plots to determine the treatment effects during the growing season. Sampling was conducted 3 days before to treatment application, and 10, 20, 30 and 50 days after treatments. Three random samples were collected from two central rows of each treatment plot, with five stems/sample. Wheat stems were cut from the base of plants with help of scissors, placed into one zipper-lock bag, and kept in picnic cooler. During the final sampling time, however, clumps of stems were pulled randomly from three sampling points of two middle rows of each plot with the help of shovel to collect entire matured plants. This technique was used mainly because the WSS diapausing larvae usually prefer to remain at the base of the wheat stem.
Samples were brought to the laboratory, where stems were dissected lengthwise with a fine bladed scalpel to determine the following parameters: 1) WSS stem infestation level; the presence of WSS immatures, parasitoid immature or frass inside dissected wheat stems, 2) WSS immatures population; the presence of eggs and larvae inside dissected wheat stems at each sampling time, 3) WSS larval mortality; the presence of dead larvae inside dissected wheat stems, 4) WSS larval body weight; body weight of diapausing larvae and 5) parasitism rate; presence of parasitoid cocoons inside stems.
Host and parasitoid adult populations: WSS and Bracon spp.
This study examined that SPDE chemicals had the ability to repel WSS adult populations and further impacts on its associated parasitoid species. A sweep net was used to estimate the insect populations. Sweeping was performed with a standard sweep net, and 15 sweeps were made from each treatment plot. The sampling was performed the 3 days before treatment and, 10, 20 and 30 days after treatments application. The samples will be stored in the freezer until to diagnosis under the lab conditions.
Stem lodging level at harvest
WSS larval feeding in stems weakened stems, lodge and cause difficulty during harvesting (Holmes 1977). This experiment examined whether SPDE chemicals had any effects on plant stand levels during the wheat grain harvest. Wheat stems lodging measurements were made by visual classification rating scale of 1 to 9. The rating of 1 indicates that all plants in a plot were vertical and 9 for all plants in a plot were horizontal.
Yield and quality
A Hege 140 plot combine was applied to harvest the wheat grains from treatment plots. The precaution was used to avoid the borders and any overlap of treatment effects on wheat yield and quality. Each plot length was measured, and the wheat grain threshed from each plot. Wheat grains were cleaned with a seed processor (Almaco, Nevada, IA) and weighed on a scale to determine yield. Test weight was measured on a Seedburo test weight scale. The protein and moisture percentages of seed were determined with NIR grain analyzer IM 9500 (Perten Instruments, Springfield, IL).
Statistical analysis
One-way-ANOVA was performed to determine the effects of SPDE chemicals treatments on WSS adults settling behaviour, WSS infestation level, WSS diapausing larval body weight, WSS diapausing larval mortality, stem lodging, and yield and quality parameters of winter wheat fields infested with WSS. Tukey test was used as a post hoc test for multiple comparisons between the means at probability (α = 0.05). Paired t-test was used to assess the WSS adult settling behavior when adults were exposed to SPDE chemicals and water sprayed plants together under choice conditions. The data was analyzed using the software statistical package R 2.15.1 (R Development Core Team, 2017).
Results
Laboratory experiments
WSS adult settling preference
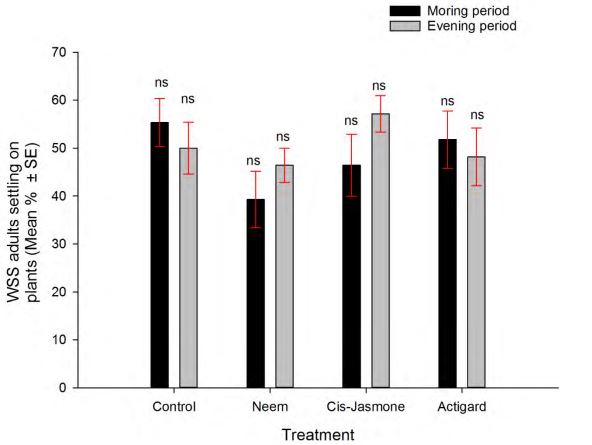
In a no- choice situation, the SPDE chemicals treatments had not shown any effect on WSS adults settling behavior (df = 3, 56; F = 1.20; P = 0.32) although there was a tendency towards the low numbers of adults located on the Neem treated plants as compared to control (water sprayed) plants (Fig 1). In addition, time had not any effect on WSS adults settling behavior (df = 1, 56; F = 1.49; P = 0.22). However, in the choice situation, there was significantly higher WSS adults settling on control than Neem treated winter wheat plants both at the morning (t = -5.79; df
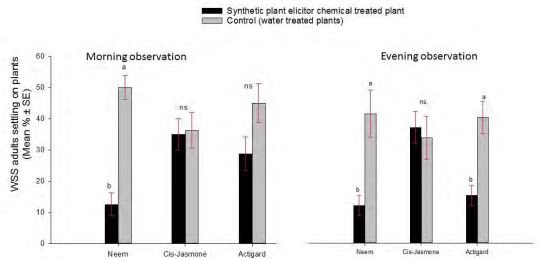
= 7; P = 0.0006712) and evening (t = -3.0; df = 7; P = 0.01935) observation periods. WSS adults also preferred significantly to the control compared to the Actigard treated plants group at the evening (t = -3.66; df = 7; P = 0.007999), but without an effect at the morning (t = -1.47; df = 7; P = 0.1834) observation period (Fig 2). In contrast, there was no significant difference between in adults settling number between the control and Cis-Jasmone treated plants groups morning (t =-0.11; df = 7; P = 0.9079) and evening (t = 0.28; df = 7; P = 0.7849) observation periods (Fig 2).
Field experiments
WSS infestation level
WSS infestation levels at different sampling time are presented at Table 1. Overall, there was high variability in infestation levels at different time of sampling. Treatments, particularly, the application of Actigard or Cis-Jasmone appeared to have some effect on infestation level at 10 days after the treatments application (Table 1).
Table 1. Effects of synthetic plant defense elicitor chemicals treatments application on WSS infestation levels in winter wheat at the three study locations of Montana, 2017.
|
Wheat stem sawfly Mean Infestation Level (%) |
|||||
|
Treatment |
PT |
10 DAT |
20 DAT |
30 DAT |
50 DAT |
|
Knees |
|
|
|
|
|
|
Water-BEL |
23 |
38 |
55 |
67 |
75 |
|
Neem-BEL |
22 |
23 |
38 |
60 |
85 |
|
Actigard-BEL |
25 |
32 |
38 |
60 |
82 |
|
Cis-BEL |
22 |
35 |
57 |
70 |
81 |
|
Actigard-AEL |
10 |
25 |
57 |
53 |
74 |
|
Water-BAEL |
20 |
42 |
65 |
71 |
72 |
|
Neem- BAEL |
18 |
25 |
50 |
55 |
78 |
|
Cis- BAEL |
20 |
17 |
48 |
55 |
81 |
|
Actigard- BAEL |
22 |
27 |
35 |
35 |
62 |
|
Neem-AEL |
18 |
33 |
48 |
63 |
77 |
|
Cis- AEL |
22 |
37 |
47 |
67 |
72 |
|
Water-AEL |
25 |
40 |
52 |
77 |
82 |
|
Choteau |
|
|
|
|
|
|
Water-BEL |
17 |
57 |
67 |
77 |
93 |
|
Neem-BEL |
22 |
48 |
60 |
78 |
93 |
|
Actigard-BEL |
20 |
37 |
55 |
80 |
95 |
|
Cis-BEL |
26 |
53 |
53 |
72 |
97 |
|
Actigard-AEL |
11 |
35 |
55 |
78 |
82 |
|
Water-BAEL |
25 |
48 |
61 |
82 |
97 |
|
Neem- BAEL |
15 |
55 |
52 |
62 |
72 |
|
Cis- BAEL |
10 |
24 |
77 |
82 |
97 |
|
Actigard- BAEL |
18 |
33 |
58 |
83 |
79 |
|
Neem-AEL |
22 |
35 |
47 |
77 |
100 |
|
Cis- AEL |
22 |
63 |
70 |
85 |
99 |
|
Water-AEL |
15 |
58 |
66 |
87 |
97 |
|
Conrad |
|
|
|
|
|
|
Water-BEL |
10 |
25 |
33 |
57 |
83 |
|
Neem-BEL |
15 |
27 |
38 |
58 |
66 |
|
Actigard-BEL |
10 |
15 |
45 |
60 |
82 |
|
Cis-BEL |
8 |
15 |
40 |
57 |
78 |
|
Actigard-AEL |
16 |
13 |
33 |
50 |
60 |
|
Water-BAEL |
27 |
28 |
45 |
58 |
79 |
|
Neem- BAEL |
12 |
15 |
33 |
53 |
56 |
|
Cis- BAEL |
17 |
37 |
32 |
52 |
57 |
|
Actigard- BAEL |
9 |
17 |
30 |
58 |
70 |
|
Neem-AEL |
10 |
23 |
45 |
68 |
84 |
|
Cis- AEL |
13 |
20 |
55 |
60 |
71 |
|
Water-AEL |
10 |
26 |
48 |
53 |
73 |
BEL: WSS Before egg laying; AEL: WSS Before egg laying; BAEL: WSS Before and after egg laying. PT: Pre-Treatment; DAT: Days after treatments.
Body weight of diapausing WSS larvae
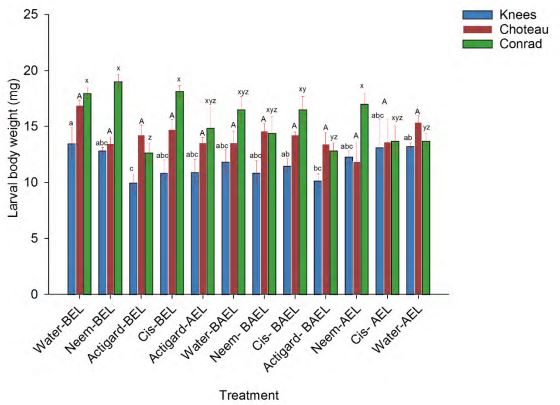
Higher body weight of diapausing larvae were usually found at the Conrad followed by Choteau and Knees locations. Mean larval body weight recorded for SPDE chemicals including controls plots ranged from 12.66-19.00 mg, 11.88-16.90 mg and 9.67-13.46 mg for Conrad, Choteau and Knees locations, respectively. This study reported that SPDE chemicals treatments had significant impact on larval body weight at the two study locations: Knees (df = 11, 36; F = 3.35; P = 0.002) and Conrad (df = 11, 35; F = 4.89; P = 0.0001), but no significant effect at the Choteau location (df = 11, 35; F = 1.85; P = 0.08) (Fig 3).
At the Knees location, among the treatment plots, significantly lower larval body weight was observed when wheat plots were treated with one time (before egg laying) or two times (before and egg laying) applications of Actigard when compared to the water (control) sprayed plots (Fig 3). There were no significant differences in larval body weights with other treatments applications. Similarly, at the Conrad location, wheat plots treated with one time application of Actigard before to WSS egg laying had lower larval body weight in comparison to the control (water sprayed) plots. Other treatments had no significant effects on larval body weight (Fig 3).
WSS diapausing larval mortality
Normally, higher diapausing WSS larval mortality was observed at the Knees followed by Conrad and Choteau locations, regardless of the treatment. Total mean larval mortality ranged 11-65 %, 12-58 % and 2-24 % for Knees, Conrad and Choteau locations, respectively. The results indicated that SPDE chemicals treatments had a significant influence on diapausing WSS larval mortality at each study location: Knees (df = 11, 36; F = 4.78; P = 0.001), Choteau (df = 11, 36; F = 3.45; P = 0.003) and Conrad (df = 11, 35; F = 2.61; P = 0.01).
Actigard treatment applications generally inflicted higher WSS diapausing larval mortality when compared to other treatments including controls, irrespective of locations. At the Knees location, wheat plots treated with one time (before or after egg laying) or two times (before and after egg laying) applications of Actigard, and one time Cis-Jasmone (after egg laying) applications were found to cause higher larval mortality when compared to the control treatments. However, one time (after egg laying) or the two times application of Actigard had only inflicted significantly higher mortality in comparisons to the water control treatments (Table 2). Similarly, at the Choteau and Conrad locations, significantly higher WSS larval mortality was recorded for the treatment with two times applications of Actigard as compared to the water control. In contrast, WSS larvae were found to be the least susceptible to Neem applications at all three locations, with no significant differences in larval mortality compared to control treatments (Table 2).
Wheat stem lodging at harvest
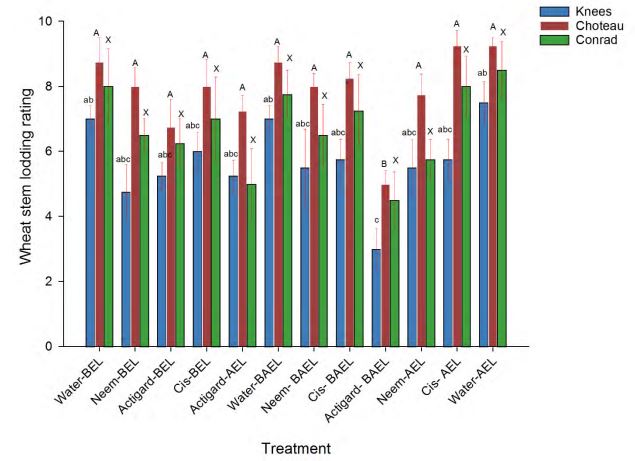
To assess the influence of SPDE chemical treatments on wheat stem lodging at harvest, the recorded visual rating stem lodged data of each SPDE chemical treatment plot was compared with the control (water) sprayed treatment plots. The results reported that the application of SPDE chemical treatments had a significant impact on wheat stem lodging at two locations: Knees (df = 11, 36; F = 3.02; P = 0.006) and Choteau (df = 11, 36; F = 4.20; P = 0.0005), but without a significant effect at the Conrad location (df = 11, 36; F = 1.78; P = 0.09).
Generally, at the Knees location, all SPDE chemical treatments were able to maintain lower stem lodging levels when compared to control treatment application. However, the stem lodged for a treatment with the two times application of Actigard was only significantly lower than the water controls (Fig 4). There was no significant differences in the lodging of the three SPDE chemicals treated wheat plots.
Regarding lodging results from Choteau location, on time (before or after WSS egg laying) or two times (before and after WSS egg laying) Actigard application provided lower lodging rating over the other treatments including controls. However, two times application of Actigard (before and after WSS egg laying) had only significantly minimal lodging rate when compared to the wheat plots treated with control (water) treatments (Fig 4).
Table 2. Effects of synthetic plant defense elicitor chemicals treatment application on total mortality of diapausing larvae (Mean ± SE), recorded in dissected stem at final harvest in winter wheat at the three study locations of Montana
|
Treatment |
Diapausing Larval Mortality (Mean ± SE) |
|
|
Knees |
Choteau |
Conrad |
|
Water-BEL |
23 ± 2.75cd |
2 ± 1.79bc |
19 ± 4.38abc |
|
Neem-BEL |
21 ± 9.77cd |
8 ± 9.48ab |
25 ± 12.12abc |
|
Actigard-BEL |
34 ± 5.99bc |
14 ± 3.78ab |
34 ± 4.37abc |
|
Cis-BEL |
18 ± 4.26cd |
13 ± 1.80ab |
23 ± 9.29abc |
|
Actigard-AEL |
46 ± 1.79abc |
11 ± 4.71ab |
13 ± 5.32abc |
|
Water-BAEL |
17 ± 3.5cd |
10 ± 2.25bc |
12 ± 2.16bc |
|
Neem- BAEL |
20 ± 4.55cd |
16 ± 2.50bc |
17 ± 0.71bc |
|
Cis- BAEL |
26 ± 7.79cd |
12 ± 4.43ab |
25 ± 13.25abc |
|
Actigard- BAEL |
65 ± 1.79ab |
33 ± 1.88a |
54 ± 7.28a |
|
Neem-AEL |
32 ± 4.55bc |
19 ± 3.53ab |
10 ± 2.78bc |
|
Cis- AEL |
36 ± 7.79abc |
23 ± 7.44ab |
27 ± 15.14abc |
|
Water-AEL |
17 ± 3.54cd |
11 ± 3.54bc |
25 ± 5.13abc |
Mean values within columns bearing the same letters within each location are not significantly different (One-way-ANOVA followed by Tukey test, P > 0.05). BEL: WSS Before egg laying; AEL: WSS Before egg laying; BAEL: WSS Before and after egg laying.
Yield
Irrespective of treatment and location, wheat plots treated with Cis-Jasmone maintained numerically higher yield levels than the Actigard applications. However, no significant differences were found between treatments at any locations (Knees: df = 11, 36; F = 0.635; P = 0.78; Choteau: df = 11, 36; F = 0.50; P = 0.89; and Conrad: df = 11, 36; F = 0.28; P = 0.98).
Average winter wheat grain yield level ranged from 54-61 bushel/acre, 55-62 bushel/acre, and 68-81 bushel/acre at the Knees, Choteau and Conrad locations, respectively (Table 3).
Quality
This study showed that treatments had no significant impact in a test weight at any studied locations: Knees (df = 11, 36; F = 1.67; P = 0.12), Choteau (df = 11, 36; F = 1.09; P = 0.40) and Conrad (df = 11, 36; F = 1.53; P = 0.16). The overall test weight was numerically higher at the Conrad location (61-64 lbs/bushel) followed by Knees (61-62 lbs/bushel) and Choteau (59-61 lbs/bushel) (Table 3). Similarly, there were no significant differences in protein % of SPDE chemicals treatments or controls at any study location: Knees (df = 11, 36; F = 0.70; P = 0.73), Choteau (df = 11, 36; F = 1.49; P = 0.17) and Conrad (df = 11, 36; F = 0.73; P = 0.69).
Numerically, protein levels were generally higher at Choteau (13-15 %) and Conrad (13-14 %) locations, while lower at the Knees (11-12 %) location (Table 3).
Table 3. Effect of synthetic plant defense elicitor chemicals treatment application on yield and quality parameters of wheat stem sawfly infested winter wheat (cv. Yellowstone) at the three study locations of Montana, 2017
|
Treatment |
Yield (bushel/acre) |
Test Weight (lbs/bushel) |
Protein Level (%) |
|
Knees Location |
|
|
|
|
Water-BEL |
58 ± 2.78a |
61 ± 0.11a |
11 ± 0.05a |
|
Neem-BEL |
60 ± 2.40a |
61 ± 0.14a |
11 ± 0.19a |
|
Actigard-BEL |
54 ± 3.66a |
62 ± 0.09a |
11 ± 0.18a |
|
Cis-BEL |
60 ± 2.24a |
61 ± 0.22a |
11 ± 0.33a |
|
Actigard-AEL |
57 ± 3.34a |
62 ± 0.11a |
12 ± 0.23a |
|
Water-BAEL |
59 ± 2.63a |
61 ± 0.34a |
11 ± 0.27a |
|
Neem- BAEL |
57 ± 3.33a |
62 ± 0.07a |
11 ± 0.21a |
|
Cis- BAEL |
61 ± 1.33a |
61 ± 0.28a |
11 ± 0.13a |
|
Actigard- BAEL |
55 ± 2.95a |
62 ± 0.20a |
12 ± 0.18a |
|
Neem-AEL |
58 ± 2.12a |
61 ± 0.14a |
11 ± 0.21a |
|
Cis- AEL |
57 ± 2.14a |
61 ± 0.03a |
11 ± 0.45a |
|
Water-AEL |
56 ± 1.90a |
61 ± 0.48a |
12 ± 0.36a |
|
Choteau Location |
|
|
|
|
Water-BEL |
54 ± 6.06a |
60 ± 0.66a |
14 ± 0.29a |
|
Neem-BEL |
55 ± 1.22a |
59 ± 0.76a |
14 ± 0.53a |
|
Actigard-BEL |
58 ± 6.30a |
59 ± 0.41a |
15 ± 0.40a |
|
Cis-BEL |
59 ± 5.69a |
59 ± 0.59a |
13 ± 1.03a |
|
Actigard-AEL |
59 ± 3.89a |
60 ± 0.13a |
15 ± 0.18a |
|
Water-BAEL |
57 ± 4.53a |
60 ± 0.59a |
13 ± 0.40a |
|
Neem- BAEL |
59 ± 2.98a |
59 ± 0.63a |
14 ± 0.31a |
|
Cis- BAEL |
62 ± 2.04a |
60 ± 0.39a |
13 ± 0.42a |
|
Actigard- BAEL |
58 ± 5.56a |
61 ± 0.09a |
14 ± 0.30a |
|
Neem-AEL |
58 ± 2.48a |
60 ± 0.57a |
14 ± 0.45a |
|
Cis- AEL |
55 ± 2.74a |
59 ± 0.60a |
15 ± 0.13a |
|
Water-AEL |
56 ± 2.30a |
60 ± 0.64a |
14 ± 0.40a |
|
Conrad Location |
|
|
|
|
Water-BEL |
65 ± 12.15a |
62 ± 0.30a |
13 ± 0.32a |
|
Neem-BEL |
74 ± 9.63a |
62 ± 0.31a |
13 ± 0.39a |
|
Actigard-BEL |
70 ± 7.14a |
62 ± 0.60a |
13 ± 0.32a |
|
Cis-BEL |
81 ± 9.73a |
64 ± 1.09a |
14 ± 0.95a |
|
Actigard-AEL |
76 ± 8.54a |
61 ± 0.51a |
13 ± 0.39a |
|
Water-BAEL |
67 ± 11.18a |
62 ± 0.50a |
13 ± 0.53a |
|
Neem- BAEL |
70 ± 8.74a |
62 ± 0.43a |
13 ± 0.47a |
|
Cis- BAEL |
74 ± 10.79a |
62 ± 0.31a |
13 ± 0.20a |
|
Actigard- BAEL |
69 ± 5.99a |
61 ± 0.53a |
13 ± 0.28a |
|
Neem-AEL |
72 ± 7.97a |
61 ± 0.55a |
13 ± 0.44a |
|
Cis- AEL |
78 ± 6.42a |
61 ± 0.39a |
13 ± 0.23a |
|
Water-AEL |
68 ± 4.72a |
61 ± 0.19a |
13 ± 0.23a |
The number of replicates per treatment was four. Mean values within columns bearing the same letters within each location are not significantly different (one-way-ANOVA followed by Tukey test, P > 0.05). BEL: WSS Before egg laying; AEL: WSS Before egg laying; BAEL: WSS Before and after egg laying.
Figure 1. Effect of synthetic plant defense elicitor chemicals treatments application on wheat stem sawfly adults settling behavior under no-choice condition.
Figure 2. Effect of synthetic plant defense elicitor chemicals treatments application on wheat stem sawfly adults settling behavior under choice condition.
Figure 3. Effect of synthetic plant defense elicitor chemicals treatments applications on the body weight of diapausing WSS larvae. Bar of each color bearing the same letters are not significantly different (One-way- ANOVA followed by Tukey test, P > 0.05).
Figure 4. Effect of synthetic plant defense elicitor chemicals treatments applications on the wheat stem lodging recorded at the harvesting time. Bar of each color bearing the same letters are not significantly different (One-way-ANOVA followed by Tukey test, P > 0.05). BEL: WSS Before egg laying; AEL: WSS Before egg laying; BAEL: WSS Before and after egg laying.
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank summer intern Bert Paulsen for assistance with field work.
References
Holmes, N. D. (1977). The effect of the wheat stem sawfly, Cephus cinctus (Hymenoptera: Cephidae), on the yield and quality of wheat. The Canadian Entomologist 109: 1591-1598.
Knodel, J. J., Beauzay, P. B., Eriksmoen, E. D., & Pederson, J. D. (2009). Pest management of wheat stem maggot (Diptera: Chloropidae) and wheat stem sawfly (Hymenoptera: Cephidae) using insecticides in spring wheat. Journal of Agricultural and Urban Entomology 26: 183-197.
Nansen, C., Macedo, T. B., Weaver, D. K., & Peterson, R. K. (2005). Spatiotemporal distributions of wheat stem sawfly eggs and larvae in dryland wheat fields. The Canadian Entomologist 137: 428-440.
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available: http://www.R-project.org. Cited 19 December 2017
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed research 14: 415-421.