Field Testing the Effect of Biopesticides against Wheat Stem Sawfly Management: Dose Response
Principal Investigator: Gadi V.P. Reddy
Project Personnel: Govinda Shrestha, Rama Devi Gadi, Debra Miller, John H. Miller and Julie Prewitt
Western Triangle Agricultural Research Center, Montana State University, 9546 Old Shelby Rd., P.O. Box 656, Conrad, MT 59425, USA
Aim of the Study
The aim of this study was: 1) to determine the effects of three biopesticides (Actigard, Xpectro and Neem) treatment application on wheat stem sawfly (WSS) management, using two doses (low and high concentration) of each product.
Materials and Methods
Locations of winter wheat fields used in field trials.
The field experiments were performed at four locations: Knees (N 48°00'08.5 W 111°21'51.8), Conrad (N 48°18'29.0 W 111°55'23.1), Devon (N 48°55' 31.1W 111°39' 64.4), and Choteau (N 47°59'36.0 W 112°06'49.9), in the Golden Triangle, Montana, USA. All experimental locations had moderate to high WSS infestations for several years. The experimental plots were seeded in September 2017 at a rate of 194 live seeds per m2. The seeds were planted in four rows, with 30 cm between rows. Glyphosate (Roundup Powermax®) was applied at the rate of 2.5 L/ ha (the active ingredient of 540 g/L of acid glyphosate) prior seeding to control weed growth. Fertilizers N, P, and K at 224.2, 0, and 22.4 kg/ha were broadcasted while planting, and an additional application of 12.3, 25.2, and 0 kg/ha of these three nutrients were applied through the seed plot drill.
At each field location, treatments were arranged in a randomized complete block design (RCBD) with nine replicates per treatment. Plots for treatments were 3.6 × 1.2 m separated by 0.60 m buffer zones to avoid cross contamination of treatments.
Monitoring of wheat stem sawfly adults.
Considering the ideal application time for biopesticides can be one of the important factors for WSS management. Currently, there is no established degree-day model for determining the precise timing of adult emergence. Two methods were used for monitoring the emergence of adults: 1) dissection of WSS-infested stubble to determine the stage of immature development and 2) sweep net sampling in the winter wheat fields to detect adults. Experimental plots and their adjacent winter wheat fields were scouted weekly from the last week of April – first week of June, 2018.
Application of chemicals
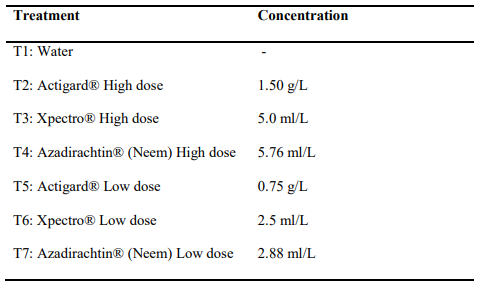
From 2017 WSS lab and field trials, we found that two-time application of two biopesticide products (Actigard and Neem) had some impacts on WSS management (Shrestha et al., 2018). In addition, 2016 WSS field trial study showed that two-time application of Xpectro biopesticide product had also some impact especially WSS larval mortality. The two-time applications refer to applications of chemicals when WSS eggs and larvae are expected to be present, respectively, inside stems. Therefore, these three chemicals were used for the study (Table 1). Since these chemicals are relatively expensive than synthetic insecticides, it is important to test whether the repeated lower doses of potential biopesticide product may work for WSS management and thereby reducing costs for winter wheat producers. The rate of each chemical is presented in Table 1.
Treatment application were based on sawfly adult’s emergence timing. First application were done on June 5 (Knees and Choteau locations) and June 6 (Devon and Conrad locations), 2018. The second application was made on June 12(Knees and Choteau locations) and June 13 (Devon and Conrad locations), 2018. Treatments were applied using a SOLO backpack sprayer (SOLO, Newport News, VA) calibrated to deliver about 400 L of spray solution/ha based on nozzle flow and walking speed. Plants treated with water served as untreated control plots. At all field trial locations, chemicals were applied at the wheat stage with 4-6 nodes.
Table 1. Biopesticide products and rate of application in each treatment
Collection of wheat stems
Wheat stems were sampled in all plots to determine the treatment effects during the growing season. Sampling was conducted 3 days before to treatment application (PT), and 10 and 50 days after treatments. Three random samples were collected from two central rows of each treatment plot, with five stems/sample. Wheat stems were cut from the base of plants with help of scissors, placed into one zipper-lock bag, and kept in picnic cooler. During the final sampling time, however, clumps of stems were pulled randomly from three sampling points of two middle rows of each plot with the help of shovel to collect entire matured plants. This technique was used mainly because the WSS diapausing larvae usually prefer to remain at the base of the wheat stem.
Samples were brought to the laboratory, where stems were dissected lengthwise with a fine bladed scalpel to determine the following parameters: 1) WSS stem infestation level; the presence of WSS immatures, parasitoid immature or frass inside dissected wheat stems, 2) WSS immatures population; the presence of eggs and larvae inside dissected wheat stems at each sampling time, 3)
WSS larval mortality; the presence of dead larvae inside dissected wheat stems, 4) WSS larval body weight; body weight of diapausing larvae and 5) parasitism rate; presence of parasitoid cocoons inside stems parasitoid holes in stems.
Host and parasitoid adult populations: WSS and Bracon spp.
Three biopesticide products were also tested to examine whether they can repel WSS adults and their impact on WSS parasitoid adult population levels. A sweep net was used to assess insect population (WSS and parasitoid adults). Sweeping was done with a standard sweep net (180o arc), collecting 15 sweeps from each treatment plot. Sampling was done one to two days before treatment application (PT) and, 10, 20 and 30 days after treatment application. Samples were stored in a freezer until examined in the laboratory and insects counted.
Stem lodging level at harvest
WSS larval feeding inside stems caused wheat stands fall into ground and thereby cause difficulty during harvesting. We examined that whether tested chemicals had any effects on plant stand levels during the wheat grain harvest. Wheat stems lodging measurements were made by visual classification rating scale of 1 to 10. The rating of 1 indicates that all plants in a plot were vertical and 10 for all plants in a plot were horizontal.
Yield and quality
To harvest the wheat grains from treatment plots, Hege 140 plot combine was used. The precaution was used to minimize the borders and any overlap of treatment effects on wheat yield and quality. Each plot length was measured, and the wheat grain threshed from each plot. Wheat grains were cleaned with a seed processor (Almaco, Nevada, IA) and weighed on a scale to determine yield. Test weight was measured on a Seedburo test weight scale. The protein and moisture percentages of seed were determined with NIR grain analyzer IM 9500 (Perten Instruments, Springfield, IL).
Statistical analysis
One-way-ANOVA was performed to determine the effects of treatments on WSS infestation level, WSS diapausing larval body weight, WSS diapausing larval mortality, parasitism level, stem lodging, and yield and quality parameters of winter wheat fields infested with WSS. Tukey test was used as a post hoc test for multiple comparisons between the means at probability (α = 0.05). A non-parametric one-way ANOVA was used to examine the effect of treatment on WSS and parasitoid adults’ population at each sampling time. Mann-Whitney U-tests were used as post hoc for multiple comparisons between treatment means. The data was analyzed using the software statistical package R 2.15.1 (R Development Core Team, 2011).
Results
WSS infestation level
WSS infestation levels at different sampling time are presented at Table 2. This study showed that treatments had no significant impacts on WSS infestation levels (see Table 2 for statistical output). Overall, there was high variation in infestation levels at different time of sampling. However, Actigard or Xpectro treatments had numerically lower infestation levels compared with untreated control at 10 days after the treatments application, irrespective of location (Table 2).
Table 2. Effects of Neem, Xpectro and Actigard applications on wheat stem sawfly (WSS) infested stem % level (mean ± SE) in winter wheat fields at the four study location of Montana.
|
WSS Infested Stem Percentage |
|||
|
Treatment |
PT |
10 DAT |
50 DAT |
|
Choteau |
|
|
|
|
Control |
3.94 ± 2.30 |
54.82 ± 7.25 |
88.87 ± 1.78 |
|
Neem Low |
9.17 ± 3.13 |
48.99 ± 8.47 |
88.49 ± 2.38 |
|
Neem High |
1.97 ± 1.23 |
57.61 ± 7.97 |
88.01 ± 3.46 |
|
Xpectro Low |
6.46 ± 2.98 |
48.37 ± 4.42 |
89.88 ± 2.56 |
|
Xpectro High |
6.01 ± 1.51 |
42.16 ± 6.71 |
90.67 ± 2.37 |
|
Actigard Low |
11.73 ± 6.85 |
41.69 ± 5.58 |
87.00 ± 3.58 |
|
Actigard High |
3.89 ± 1.47 |
56.24 ± 7.76 |
88.84 ± 2.64 |
|
Stat. output |
F6, 49 = 0.91; P =0.49 |
F6, 56 = 0.84; P =0.54 |
F6, 56 = 0.17; P =0.98 |
|
Knees |
|
|
|
|
Control |
12.29 ± 3.60 |
69.83 ± 5.13 |
90.47 ± 3.33 |
|
Neem Low |
14.12 ± 5.95 |
65.30 ± 5.68 |
90.19 ± 4.45 |
|
Neem High |
12.86 ± 4.76 |
71.00 ± 9.25 |
94.79 ± 2.48 |
|
Xpectro Low |
16.65 ± 3.60 |
55.14 ± 7.10 |
82.85 ± 4.81 |
|
Xpectro High |
14.82 ± 2.03 |
52.03 ± 8.54 |
89.76 ± 3.60 |
|
Actigard Low |
17.07 ± 3.80 |
67.74 ± 7.28 |
85.61 ± 5.11 |
|
Actigard High |
16.68 ± 3.52 |
65.01 ± 6.63 |
84.27 ± 3.23 |
|
Stat. output |
F6, 49 = 0.21; P =0.97 |
F6, 56 = 1.16; P =0.34 |
F6, 56 = 0.42; P =0.42 |
|
Devon |
|
|
|
|
Control |
9.58 ± 4.33 |
44.85 ± 8.36 |
51.78 ± 5.42 |
|
Neem Low |
4.84 ± 2.31 |
41.03 ± 4.94 |
47.77 ± 7.72 |
|
Neem High |
7.98 ± 3.93 |
37.57 ± 8.25 |
54.96 ± 7.98 |
|
Xpectro Low |
5.06 ± 2.76 |
34.64 ± 7.05 |
51.03 ± 4.92 |
|
Xpectro High |
1.85 ± 1.15 |
35.13 ± 4.78 |
53.86 ± 6.46 |
|
Actigard Low |
5.12 ± 1.95 |
34.95 ± 9.18 |
51.03 ± 4.21 |
|
Actigard High |
4.03 ± 2.71 |
29.63 ± 6.24 |
46.90 ± 2.81 |
|
Stat. output |
F6, 49 = 0.72; P =0.64 |
F6, 56 = 1.16; P =0.33 |
F6, 56 = 0.25; P =0.95 |
|
Conrad |
|
|
|
|
Control |
13.47 ± 4.44 |
32.32 ± 7.85 |
75.99 ± 4.41 |
|
Neem Low |
11.59 ± 3.29 |
30.79 ± 6.20 |
80.31 ± 4.46 |
|
Neem High |
16.27 ± 5.09 |
38.57 ± 3.85 |
81.21 ± 2.91 |
|
Xpectro Low |
19.90 ± 6.04 |
31.81 ± 3.67 |
74.15 ± 4.25 |
|
Xpectro High |
8.80 ± 3.31 |
33.26 ± 7.00 |
80.99 ± 2.69 |
|
Actigard Low |
9.51 ± 2.62 |
28.07 ± 6.62 |
78.25 ± 3.68 |
|
Actigard High |
16.00 ± 2.32 |
14.14 ± 3.03 |
75.48 ± 3.21 |
|
Stat. output |
F6, 49 = 0.88; P =0.51 |
F6, 56 = 1.78; P =0.12 |
F6, 56 = 0.59; P =0.73 |
PT, Pre Treatment; DAT, Days After Treatment Application
Wheat stem sawfly adults, and parasitoid adults and their parasitism level
In general, WSS adult populations were found higher at the Knees location followed by the Choteau, Conrad and Devon locations (Table 3). Regardless of location, treatments did not have a significant impact on WSS adult population, at any sampling time (see Table 3 for statistical output). Except, at the Knees location, the wheat plots treated with lower dose of Xpectro had significantly lower WSS adult population compared with the untreated control plots at 10 days after treatment application (Table 3).
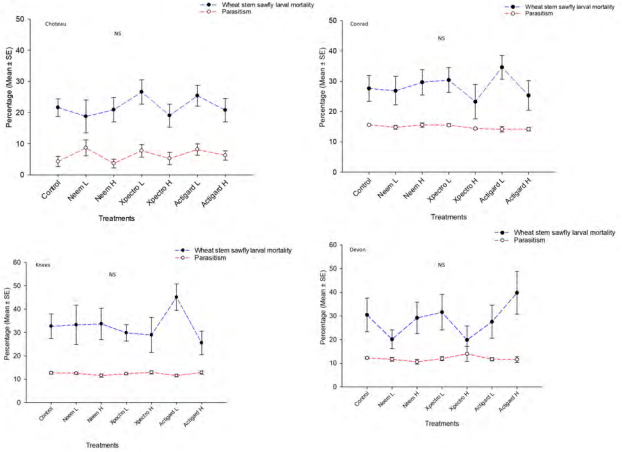
Regarding Bracon spp. adult, there were no significant differences in parasitoid adult population levels between treatments at each sampling time at the Choteau, Knees, Devon and Conrad locations (Table 3). In addition, the study did not depict any significant difference on parasitism levels between treatments either of study locations (Choteau; df = 6, 56; F = 1.13; P = 0.36; Conrad: df = 6, 56; F = 0.67; P = 0.68, Knees; df = 6, 56; F = 0.89; P = 0.50 and Devon; df = 6, 56; F = 1.48; P = 0.20). (Figure 1). The overall average parasitism at the Choteau, Knees, Devon and Conrad locations varied from 3.61-8.62 %, 11.50 -12.81 %, 10.69-12.28 and 14.39-15.61 %,
respectively (Figure 1).
WSS diapausing larval mortality
In overall, higher diapausing WSS larval mortality was observed at the Knees followed by Devon, Conrad and Choteau locations, irrespective of treatment. Total mean larval mortality percentage varied from 26-45, 20-40, 20-40 and 19-27 for Knees, Devon, Conrad, and Choteau locations, respectively (Figure 1). In Knees and Conrad locations, wheat plots treated with Actigard lower dose inflicted noticeably higher percentage of mortality compared to plots treated with Xpectro, Neem and control, while with higher Actigard dose application at the Devon location. However, no significant differences were found between treatments either of these locations (Knees; df = 6, 56; F = 0.98; P = 0.45; Conrad: df = 6, 56; F = 0.67; P = 0.67 and Devon; df = 6, 56; F = 1.00; P
= 0.43). Similarly, the treatments had no significant impact on larval mortality at the Choteau location (df = 6, 56; F = 0.63; P = 0.70).
Figure 1. Effect of Neem, Xpectro and Actigard applications on total mortality of diapausing larvae (mean ± SE) and parasitism levels, recorded in dissected stems and emergence holes in stems at final harvest in winter wheat at the four study locations of Montana. NS refers to non-significant.
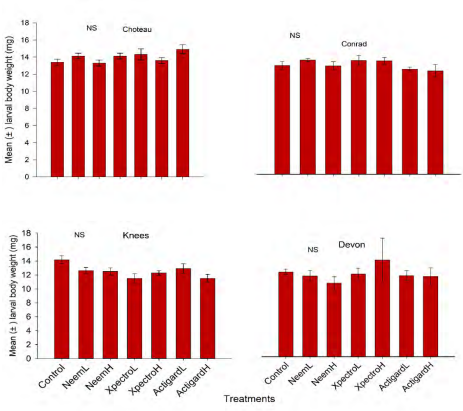
Body weight of diapausing WSS larvae
Higher body weight of diapausing larvae were generally found at the Conrad followed by Choteau Knees and Devon locations (Figure 2). Mean larval body weight recorded for treatments including control plots ranged from 14.16-15.62 mg, 13.31-14.32 mg, 11.51-14.17 mg and 10.63-14.00 mg for Conrad, Choteau, Knees and Devon locations, respectively (Figure 2). This study reported that treatments had no significant impact on larval body weight either of the study location: Conrad (df = 6, 56; F = 1.16; P = 0.34), Choteau (df = 6, 56; F = 1.52; P = 0.19), Knees (df = 6, 56; F = 0.98; P = 0.45) and Devon (df = 6, 56; F = 0.57; P = 0.75) locations (Figure 2).
Figure 2. Effect of Neem, Xpectro and Actigard applications on diapausing larvae (mean ± SE) body weight, recorded in dissected stems at final harvest in winter wheat at the four study locations of Montana. NS refers to non-significant.
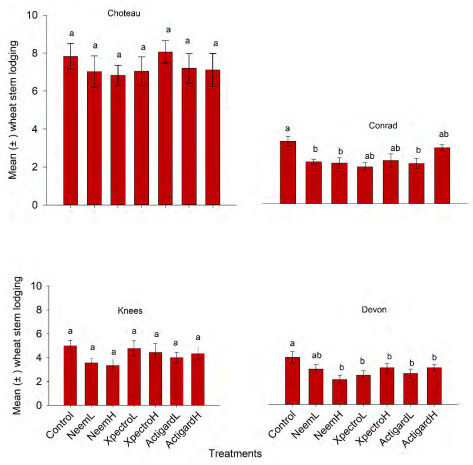
Wheat stem lodging
This study demonstrated main significant effect of treatment on wheat stem lodging at two of the study locations: Conrad (df = 11, 36; F = 3.02; P = 0.006) and Devon (df = 11, 36; F = 3.02; P = 0.006) (Figure 3). In contrast, treatments had not shown any significant effects on other two locations: Choteau (df = 11, 36; F = 0.40; P = 0.88) and Knees (df = 11, 36; F = 1.22; P = 0.31). At Conrad location, significantly lower mean stem lodging (± SE) occurred when wheat plots were treated with Actigard lower dose (2.78 ± 0.79), and Neem low (2.89 ± 0.20) and high (2.82 ± 0.36) doses compared with untreated control plots (4.33 ± 0.33) (Figure 3). In contrast, the remaining treatments showed no significant differences from the untreated control. At the Devon location, Neem applied with higher dose had only significantly lower mean stem lodging (1.89 ± 0.31) compared with untreated control plots (3.56 ± 0.44) (Figure 3).
Yield
In overall, higher average winter wheat yield was found in Choteau (64-75 bushel/acre) followed by Knees (54-64 bushel/acre), Conrad (52-64 bushel/acre) and Devon (40-46 bushel/acre) locations. This study reported that treatments had significant impacts on winter wheat grain yield at the Choteau and Conrad locations, but without effects at the Knees and Devon locations (see Table 3 for statistical outputs). At the both Choteau and Conrad locations, wheat plots treated with higher dose of Actigard had significantly or numerically lower grain yield compared with other treatments including water (Table 4). Similar patterns were also observed at the Knees and Devon locations.
Figure 3. Effect of Neem, Xpectro and Actigard applications on On the wheat stem lodging (mean
± SE) recorded at the harvesting time at the four study location of Montana. In y-axis scale, 1 demonstrates that all plants in a plot were vertical and 10 for all plants in a plots were horizontal during harvest. Mean values within bars bearing the different letters are significantly different (Tukey test, P<0.05)
Quality
The treatments had no significant impact on a test weight at any study locations (see Table 3 for statistical output). The overall test weight was numerically higher at the Conrad (61-62 lbs/bushel) followed by Knees (60-61 lbs/bushel), Devon (59-60 lbs/bushel) and Choteau (58-59 lbs/bushel) (Table 4). Similarly, there were no significant differences in moisture percentage and 1000 kernels weight among treatments (Table 4). However, regarding to protein levels, they were significantly higher when wheat plots were treated with higher dose of Actigard compared with plots treated with water, Neem or Xpectro at the Choteau and Devon locations, but without significant effects at the Knees and Conrad locations (Table 4).
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank summer interns Bert Paulsen and Sindhu Mettupalli for assistance with field work.
References
R Development Core Team. 2011. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Shrestha, G, Briar, S.S., Reddy, G.V.R., 2018. Plant defense elicitors: Plant fitness versus wheat stem sawfly. PeerJ, DOI 10.7717/peer.5892.
Table 3. Effect of Neem, Xpectro and Actigard applications on wheat stem sawfly and Bran spp. adult individuals (15 sweeps/plot) at the four study location of Montana.
|
|
|
WSS adult number |
|
|
|
Parasitoid adult number |
|
|
|
Treatment |
PT |
10 DAT |
20 DAT |
30 DAT |
PT |
10 DAT |
20 DAT |
30 DAT |
|
Choteau |
|
|
|
|
|
|
|
|
|
Control |
15.00 ± 2.11 |
9.22 ± 2.28 |
1.333 ± 0.50 |
- |
- |
- |
0.56 ± 0.24 |
0.11 ± 0.11 |
|
Neem Low |
15.63 ± 2.26 |
9.67 ± 1.85 |
1.444 ± 0.44 |
- |
- |
- |
0.89 ± 0.26 |
0.44 ± 0.34 |
|
Neem High |
15.38 ± 1.49 |
8.00 ± 1.40 |
1.125 ± 0.52 |
- |
- |
- |
0.38 ± 0.25 |
0.11 ± 0.11 |
|
Xpectro Low |
16.63 ± 2.03 |
8.44 ± 1.76 |
1.222 ± 0.58 |
- |
- |
- |
0.11 ± 0.11 |
0.33 ± 0.17 |
|
Xpectro High |
16.38 ± 1.69 |
8.56 ± 1.36 |
2.00 ± 0.43 |
- |
- |
- |
0.44 ± 0.18 |
0.22 ± 0.15 |
|
Actigard Low |
15.63 ± 1.94 |
9.22 ± 1.76 |
2.00 ± 0.64 |
- |
- |
- |
0.33 ± 0.24 |
0.22 ± 0.15 |
|
Actigard High |
17.00 ± 2.66 |
9.67 ± 1.41 |
1.5 ± 0.60 |
- |
- |
- |
0.75 ± 0.15 |
0.11 ± 0.11 |
|
Stat. output |
χ26=0.64; P=0.10 |
χ26=1.38; P=0.97 |
χ26=3.96; P=0.68 |
|
|
|
χ26=10.32; P=0.12 |
χ26=2.44; P=0.87 |
|
Knees |
|
|
|
|
|
|
|
|
|
Control |
40.13 ± 5.34 |
7.67 ± 0.94ab |
0.11 ± 0.12 |
- |
- |
0.44 ± 0.24 |
0.22 ± 0.15 |
0.22 ± 0.15 |
|
Neem Low |
48.13 ± 7.07 |
9.11 ± 1.46a |
0.11 ± 0.12 |
- |
- |
0.00 ± 0.00 |
0.33 ± 0.17 |
0.22 ± 0.15 |
|
Neem High |
46.25 ± 4.98 |
9.78 ± 0.96a |
0.11 ± 0.12 |
- |
- |
0.33 ± 0.17 |
0.56 ± 0.29 |
0.11 ± 0.11 |
|
Xpectro Low |
42.75 ± 6.34 |
3.56 ± 0.56bc |
0.11 ± 0.12 |
- |
- |
0.00 ± 0.00 |
0.44 ± 0.24 |
0.44 ± 0.24 |
|
Xpectro High |
51.88 ± 7.67 |
8.22 ± 1.89ab |
0.11 ± 0.12 |
- |
- |
0.11 ± 0.11 |
0.44 ± 0.34 |
0.33 ± 0.17 |
|
Actigard Low |
45.88 ± 9.50 |
6.67 ± 1.83bc |
0.56 ± 0.36 |
- |
- |
0.22 ± 0.15 |
0.56 ± 0.34 |
0.33 ± 0.17 |
|
Actigard High |
52.75 ± 7.54 |
6.78 ± 0.93bc |
0.11 ± 0.12 |
- |
- |
0.33 ± 0.24 |
0.22 ± 0.15 |
0.22 ± 0.15 |
|
Stat. output |
χ26=2.38; P=0.88 |
χ26=18.09; P=0.001 |
χ26=3.40; P=0.76 |
- |
- |
χ26=7.37; P=0.28 |
χ26=1.16; P=0.97 |
χ211=2.14; P=0.91 |
|
Devon |
|
|
|
|
|
|
|
|
|
Control |
10.63 ± 1.45 |
1.22 ± 0.49 |
- |
- |
- |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
|
Neem Low |
10.13 ± 1.54 |
1.13 ± 0.48 |
- |
- |
- |
0.11 ± 0.11 |
0.11 ± 0.11 |
0 .00 ± 0.00 |
|
Neem High |
10.50 ± 2.01 |
1.56 ± 0.50 |
- |
- |
- |
0 .00 ± 0.00 |
0.22 ± 0.15 |
0 .00 ± 0.00 |
|
Xpectro Low |
11.50 ± 1.2 |
1.33 ± 0.35 |
- |
- |
- |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
0.11 ± 0.11 |
|
Xpectro High |
12.00 ± 1.98 |
1.11 ± 0.57 |
- |
- |
- |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
0.00 ± 0.00 |
|
Actigard Low |
12.88 ± 1.91 |
1.78 ± 0.42 |
- |
- |
- |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
0.22 ± 0.15 |
|
Actigard High |
8.00 ± 1.25 |
1.44 ± 0.53 |
- |
- |
- |
0 .00 ± 0.00 |
0 .00 ± 0.00 |
0.00 ± 0.00 |
|
Stat. output |
χ26=4.09; P=0.54 |
χ26=3.04; P=0.80 |
- |
- |
- |
χ26=6; P=0.42 |
χ26=8.96; P=0.18 |
χ211=8.96; P=0.17 |
|
Conrad |
|
|
|
|
|
|
|
|
|
Control |
15.63 ± 2.21 |
4.56 ± 0.83 |
0.00 ± 0.00 |
- |
- |
0.11 ± 0.11 |
0.00 ± 0.00 |
0.78 ± 0.36 |
|
Neem Low |
10.25 ± 2.12 |
4.33 ± 1.44 |
0.00 ± 0.00 |
- |
- |
0.11 ± 0.11 |
0.00 ± 0.00 |
0.78 ± 0.36 |
|
Neem High |
15.50 ± 2.46 |
4.00 ± 1.05 |
0.00 ± 0.00 |
- |
- |
0.00 ± 0.00 |
0.22 ± 0.22 |
0.33 ± 0.17 |
|
Xpectro Low |
10.63 ± 1.64 |
3.11 ± 0.60 |
0.00 ± 0.00 |
- |
- |
0.00 ± 0.00 |
0.33 ± 0.17 |
0.22 ± 0.15 |
|
Xpectro High |
10.38 ± 1.32 |
3.78 ± 0.75 |
0.11 ± 0.12 |
- |
- |
0.11 ± 0.11 |
0.00 ± 0.00 |
0.44 ± 0.18 |
|
Actigard Low |
11.38 ± 1.49 |
4.67 ± 1.16 |
0.00 ± 0.00 |
- |
- |
0.00 ± 0.00 |
0.22 0.15 |
0.44 ± 0.18 |
|
Actigard High |
9.00 ± 2.06 |
4.22 ± 0.61 |
0.00 ± 0.00 |
- |
- |
0.00 ± 0.00 |
0.33 ± 0.17 |
0.33 ± 0.24 |
|
Stat. output |
χ26=7.93; P=0.24 |
χ26=3.06; P=0.80 |
χ26=6; P=0.42 |
- |
- |
χ26=6; P=0.42 |
χ26=9.80; P=0.13 |
χ26=2.96; P=0.81 |
PT, Pre Treatment; DAT, Days After Treatment Application. Mean values within columns bearing the different letters are significantly differetn (Mann-Whitney test, P<0.05)
Table 4. Effect of Neem, Xpectro and Actigard on average yield and quality (± SE) parameter of winter wheat fields infested at the four study location of Montana
|
Treatment |
Yield (bushel/acre) |
Test weight (lbs/bushel) |
Protein (%) |
Moisture (%) |
Thousand Kernel Weight (gram) |
|
Knees |
|
|
|
|
|
|
Water |
53.80 ± 1.51 |
60.99 ± 0.13 |
13.89 ± 0.10 |
10.42 ± 0.04 |
29.77 ± 0.58 |
|
Neem L |
61.55 ± 2.30 |
61.06 ± 0.12 |
13.65 ± 0.11 |
10.37 ± 0.02 |
30.52 ± 0.47 |
|
Neem H |
59.27 ± 1.77 |
60.81 ± 0.15 |
13.69 ± 0.09 |
10.43 ± 0.05 |
30.98 ± 0.54 |
|
Xpectro L |
62.95 ± 2.43 |
60.70 ± 0.18 |
13.70 ± 0.10 |
10.45 ± 0.07 |
29.74 ± 0.60 |
|
Xpectro H |
59.40 ± 2.66 |
60.71 ± 0.15 |
13.74 ± 0.08 |
10.40 ± 0.06 |
30.24 ± 0.35 |
|
Actigard L |
55.59 ± 2.08 |
61.27 ± 0.12 |
14.02 ± 0.09 |
10.40 ± 0.04 |
30.48 ± 0.55 |
|
Actigard H |
54.65 ± 2.36 |
61.16 ± 0.16 |
14.15 ± 0.09 |
10.37 ± 0.04 |
29.38 ± 0.44 |
|
Stat. output |
F6, 50 = 2.27; P =0.06 |
F6, 56 = 1.74; P = 0.128 |
F6, 56 = 1.85; P = 0.106 |
F6, 56 = 0.40; P =0.878 |
F6, 56 = 1.59; P =0.169 |
|
Choteau |
|
|
|
|
|
|
Water |
73.28 ± 1.30a |
59.37 ± 0.24 |
14.54 ± 0.08b |
9.66 ± 0.05 |
26.97 ± 0.47 |
|
Neem L |
73.29 ± 1.56a |
59.26 ± 0.14 |
14.55 ± 0.06ab |
9.85 ± 0.04 |
26.86 ± 0.36 |
|
Neem H |
69.37 ± 2.16a |
58.87 ± 0.18 |
14.69 ± 0.11ab |
9.79 ± 0.09 |
25.97 ± 0.33 |
|
Xpectro L |
71.47 ± 1.48a |
58.93 ± 0.27 |
14.58 ± 0.07ab |
9.72 ± 0.04 |
26.63 ± 0.39 |
|
Xpectro H |
74.99 ± 1.89a |
59.47 ± 0.14 |
14.50 ± 0.08b |
9.74 ± 0.03 |
26.56 ± 0.31 |
|
Actigard L |
66.67 ± 1.12ab |
59.34 ± 0.13 |
14.96 ± 0.10a |
9.81 ± 0.07 |
25.96 ± 0.20 |
|
Actigard H |
63.71 ± 1.76bc |
59.81 ± 0.15 |
14.98 ± 0.10a |
9.76 ± 0.04 |
26.80 ± 0.34 |
|
Stat. output |
F6, 49 = 5.44; P =0.0002 |
F6, 56 = 1.83; P = 0.109 |
F6, 56 = 4.40; P = 0.001 |
F6, 56 = 1.0; P = 0.435 |
F6, 56 = 1.86; P =0.104 |
|
Devon |
|
|
|
|
|
|
Water |
40.11 ± 3.21 |
59.98 ± 0.34 |
11.78 ± 0.27c |
10.56 ± 0.10 |
26.41 ± 0.65 |
|
Neem L |
45.90 ± 2.35 |
59.58 ± 0.21 |
12.02 ± 0.24abc |
10.56 ± 0.13 |
25.93 ± 0.52 |
|
Neem H |
41.85 ± 3.18 |
59.88 ± 0.33 |
11.97 ± 0.32abc |
10.57 ± 0.15 |
26.27 ± 0.79 |
|
Xpectro L |
46.31 ± 1.94 |
59.93 ± 0.31 |
11.59 ± 0.27c |
10.60 ± 0.17 |
26.27 ± 0.76 |
|
Xpectro H |
43.08 ± 1.01 |
59.61 ± 0.36 |
11.75 ± 0.31c |
10.46 ± 0.09 |
25.97 ± 0.69 |
|
Actigard L |
41.67 ± 1.93 |
60.30 ± 0.28 |
12.44 ± 0.27ab |
10.65 ± 0.20 |
26.87 ± 0.66 |
|
Actigard H |
40.00 ± 2.80 |
60.38 ± 0.24 |
12.75 ± 0.24ab |
10.75 ± 0.21 |
26.67 ± 0.50 |
|
Stat. output |
F6, 49 = 0.95; P = 0.468 |
F6, 56 = 0.9; P = 0.502 |
F6, 56 = 2.58; P = 0.028 |
F6, 56 = 0.43; P = 0.855 |
F6, 56 = 0.43; P =0.966 |
|
Conrad |
|
|
|
|
|
|
Water |
60.62 ± 2.38abc |
61.93 ± 0.18 |
14.00 ± 0.19 |
9.38 ± 0.10 |
34.17 ± 0.47 |
|
Neem L |
63.90 ± 2.23ab |
61.93 ± 0.18 |
13.88 ± 0.20 |
9.36 ± 0.10 |
34.41 ± 0.52 |
|
Neem H |
61.63 ± 2.59abc |
61.80 ± 0.15 |
14.11 ± 0.20 |
9.41 ± 0.10 |
33.00 ± 0.90 |
|
Xpectro L |
64.35 ± 3.30ab |
61.42 ± 0.28 |
13.97 ± 0.15 |
9.22 ± 0.12 |
33.93 ± 0.55 |
|
Xpectro H |
56.27 ± 2.60abc |
61.69 ± 0.24 |
14.10 ± 0.16 |
9.20 ± 0.10 |
33.83 ± 0.59 |
|
Actigard L |
56.62 ± 2.74abc |
61.87 ± 0.18 |
14.16 ± 0.21 |
9.06 ± 0.09 |
33.46 ± 0.37 |
|
Actigard H |
51.71 ± 1.77c |
61.71 ± 0.19 |
14.52 ± 0.19 |
9.21 ± 0.15 |
33.52 ± 0.41 |
|
Stat. output |
F6, 49 = 2.88; P = 0.017 |
F6, 56 = 0.77; P = 0.595 |
F6, 56 = 0.99; P = 0.437 |
F6, 56 = 1.05; P = 0.403 |
F6, 56 = 0.64; P =0.695 |
Mean values within columns bearing the different letters are significantly differetn (Tukey test, P<0.05)