Role of entomopathogenic nematodes for the management of wireworms (Coleoptera: Elateridae)
Principal Investigator: Dr. Gadi VP Reddy
Project collaborators: Dr. David Shapiro and Dr. Byron Adams
Project personnel: Ramandeep Kaur Sandhi, Dr. Anamika Sharma, Ramadevi Gadi and Deb Miller
Montana State University, Western Triangle Agricultural Research Center, Conrad MT, 59425
Aim of the study
The different aims of the study were: (1) To test the efficacy of available EPN strains against wireworms, (2) To extract and identify the native EPN strains in Golden Triangle Area of Montana, and (3) To test the efficacy of found native EPN strains against wireworms.
Materials and methods
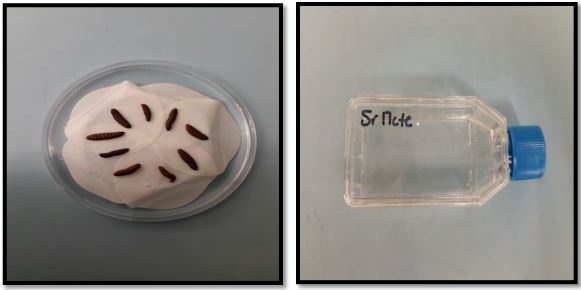
Wireworm Collection: The larvae of different instars were collected from different locations (Conrad, Pendroy, and Kallispell fields). The wireworms were collected by using stocking traps. The stocking traps with soaked wheat seeds were put in the different spots in soil and then covered with plastic sheets. After 15-20 days, the stocking traps were collected and brought back to the laboratory. These stocking traps were replaced every time we collected the old traps. The stocking traps with wireworms were put in the Berlese Funnels for 12 hours and the wireworms were collected and categorized into small, medium and large based on their size (Figure 1). We found mainly three wireworm species viz. Limonius californicus, Hypnoides bicolor, and Aeolus mellillus. However, the wireworm specie, L. californicus is the dominant specie in Golden Triangle Area of Montana, only this specie is being used in the experiments. These wireworms were stored in the incubator in the plastic cups with sterilized soil and wheat seeds as their food. These plastic cups were put in the incubator at 8° C. The wireworms were also collected manually by collecting the soil from fields infested with wireworms.
-
Efficacy of available EPN strains against wireworms:
EPN’s species: Ten nematode strains were obtained from Dr. David Shapiro (USDA ARS, Georgia). The list is given in Table 1as follows:
Table 1. List of EPN strains (Source: Dr. David Shapiro, USDA-ARS, Georgia)
Entomopathogenic nematode species
Strain
Steinernema carpocapsae
All strain
Cxrd strain
Steinernema feltiae
SN strain
Heterorhabditis bacteriophora
HP88 strain
VS strain
Steinernema riobrave
355 strain
7-12 strain
Heterorhabditis floridensis
K22 strain
Heterorhabditis georgiana
Kesha strain
Steinernema rarum
17 c + e strain
Nematodes rearing: The nematodes received were reared on waxworm larvae. Ten waxworm larvae were placed on the filter paper in the petri dishes. 500-1000 Infective Juveniles (IJ)/ml were inoculated in the petri dishes with 100-200 IJ/larva. Petri dishes were left at room temperature for nematode infection. After 3-5 days, nematodes infected larvae were placed on the white traps for rearing (Figure 1). After 7 to 10 days, nematodes were collected from the white traps and stored in the tissue culture flasks.
Figure 1. Infected waxworm larvae on white trap and collected IJs stored in tissue culture flasks
Nematode doses: For preparing the different nematode doses, the nematode were dissolved in distilled water (100-1000 ml). After making the solution, 1 ml of the nematode suspension was taken in the nematode counting slide and the number of nematodes were counted under the microscope. This was repeated 5 times for avoiding the errors in the counting. Then the number of IJ/ml were characterized. First, the higher dose i.e. 5600 IJ/ml was prepared and on the basis of this dose, further dilutions like 2800, 1400, and 700 IJ/ml were made. This procedure was followed for the all the available nematode strains. These nematode doses were stored in the incubator with 8° C temperature and used within 10 days after collection. The nematodes were observed under microscope for their survival and nematodes with >95% survival rate were used in the experiment.
Laboratory bioassay: In the bioassay, medium sized larvae (10-20 mm) of L. californicus were used. 1 oz. plastic cups were filled with 20 g of sterilized sandy loam soil (sand-78 %, silt-12%, clay-10%, pH-7.7, and organic matter-1.4) and few soaked wheat seeds were provided as a food source for the larvae. One wireworm larva which had been held at 8° C were placed in the cups. The different nematode doses (i.e. 5600, 2800, 1400, and 700 IJ/ml) were inoculated onto the soil surface in 1 ml of water. The soil moisture was standardized to 15%. In the cups with control treatment, only 1 ml of water was applied. The cups were capped with some holes and then placed in the growth chamber at 22° C, 75 % RH in the dark (Figure 2). The larval mortality was observed at 24 hr intervals for 28 days. The dead larvae were dissected under the stereomicroscope to confirm that the infection was due to the nematodes. The same experiment was repeated 5 times with different cultures of nematodes.
Penetration Rate: The penetration rate of different EPNs was also observed. The dead wireworm larvae were placed on room temperature for 2 more days after their death to let infective juveniles grow to males and females and then number of nematodes inside the larval body were counted by dissecting the larva.
Figure 2. Inoculated cups in incubator
Shade house screening: Six promising strains found from the laboratory bioassay were screened in a shade house experiment. The plastic pots (18 cm height, 12 cm diameter; surface area 600 cm sq.) were filled with sterilized sandy loam field collected soil to 10 cm height. Approximately, 8- 10 wheat seeds were sown in each plastic pot. The plants were watered twice a day because of the sandy soil (loose moisture quickly) and plants were allowed to grow for 3 weeks. After that, 5 wireworm larvae were released in the soil in each replicate. After 5 hours, 2 doses; 60000 IJs (100 IJs/cm2) and 15000 IJs (25 IJs/cm2) of promising EPN’s from laboratory (Sr 355, Sr 17 c+e, ScA11, ScCxrd, HbVS, and Sr 7-12) were inoculated in the pots with a pipette. The pots were kept in the shade house with average temperature of 21°C (5°-30°C) and soil moisture 25±5%. There were 16 replications for each strain. The larval mortality was observed after 7, 14, 21, and 28 days. 4 out of 16 replications were observed destructively and observed for larval mortality every week. The alive wireworm larvae were kept for 3 more days to observe the mortality.
-
To extract and identify the native EPN strains in Golden Triangle Area of Montana
Collection of soil samples: The soil samples were collected from 30 different fields from Pendroy, Choteau, Valier, Conrad, Kallispell, Knees, Brady, Collins, Dutton, Shelby, Sunburst, and Tiber areas were collected in Golden Triangle Area of Montana in summer 2018. The fields surveyed were winter wheat, canola, peas, alfalfa, chickpea, flax, hemp, durum wheat, lentil, triticale, spring wheat, and fallow. From each field, 5 random plots (8-10 m2) were chosen and, within each sample plot, 5 random 10-15 cm deep soil samples were taken with the help of hand shovel. Overall, there were 25 soil samples for each field site. The shovel was washed with water and sterilized with 75% ethanol in between the samples for avoiding the contamination. These collected soil samples were then placed into plastic bags brought back to laboratory in a cooler (8-10° C). The latitude, longitude, site, date, habitat, soil texture, pH, organic matter and electrical conductivity were recorded for each field soil.
Isolation of entomopathogenic nematodes; Insect Bait Method - Bedding and Akhurst (1975): Approximately 300 g soil sample was placed in 500 ml plastic cups with five G. mellonella larvae in each cup with some moisture. The plastic cups were incubated at room temperature for 7 days by observing them regularly after 2 days. If no larvae were dead after 7 days, we considered that entomopathogenic nematodes are absent from that sample. The dead G. mellonella larvae were removed and placed on the white traps. These white trap petri dishes were checked daily for nematodes. After 7 to 10 days, emerging nematodes from well infected cadavers were concentrated and checked again for G. mellonella larval mortality by inoculating with the emerged nematodes. These emerged nematodes were identified by using morphological and molecular techniques.
Morphological identification: The extracted nematode infective juveniles from different fields were cultured through Galleria mellonella. 10 Galleria larvae were infected with one hundred (100-200) nematode infective juveniles for each test isolate. The cadavers were dissected in ringer’s solution according to procedure given aby Kaya and Stock (1997). The 1st generation males and females were recovered 2-3 days after infection and others on 5-6 days for the 2nd generation of the both sexes in case of Steinernema sp.. For Heterorhabditis sp.; dissections were done 3-4 days after infection to recover 1st generation hermaphrodites, and 6-9 days after infection for 2nd generation males and females. The third stage nematode infective juveniles were collected during the 3 days after initial emergence. Overall, twenty 1st and 2nd generation adults and twenty infective juveniles were observed. The nematodes were killed in hot water at 60°C and fixed in equal amount of hot TAF (60°C) and ringer’s solution. The nematodes were left in the fixative for 2 days. After processing to glycerin in desiccator as explained by Kaya and Stock (1997), the nematodes were mounted on the glass slides and observed for different morphological characters; body length (TBL), maximum body width (MBW), tail length (TL), anal body width (ABW), distance from the anterior end to oesophagus (ES), distance from anterior end to excretory pore (EP), distance from anterior end to nerve ring (NR), spicule length (SPL) and Gubernaculum length (GUL) for males and for females, distance from anterior end to vulva (AV) including all the characters studied for males. The infective juveniles (IJs) were also observed for total body length, maximum body width, tail length, anal body width, distance from the anterior end to oesophagus, distance from anterior end to excretory pore, and distance from anterior end to nerve ring.
Molecular identification:
DNA of different EPN strains were extracted by DNeasy® DNA extraction method by following the modifications of Adams et al. (2007). Approximately 0.25 µl pellet of IJs of different strains was prepared in 2 ml microcentrifuge tubes and IJs in the tube were crushed with the pipette end. 180 µl Buffer ATL was added in the tubes followed by 20 µl proteinase K. The mixture was mixed thoroughly by vortexing, and then incubate at 60°C for 2-3 hours while vortexing every 15 min, until the tissue was completely lysed. After that, 200 µl Buffer AL was added to the sample, and mixed thoroughly by vortexing. It was followed by addition of 200 µl ethanol (100%), and mixed again thoroughly by vortexing. This mixture was pipetted into the DNeasy mini spin column placed in a 2 ml collection tube and centrifuge at 8000 rpm for 1 min. The DNeasy mini spin column was placed in a new 2 ml collection tube and 500 µl Buffer AW1 was added, and centrifuged for 1 min at 8000 rpm. Again, the DNeasy mini spin column was placed in a new 2 ml collection tube and 500 µl Buffer AW2 was added followed by centrifugation for 3 min at 14,000 rpm. Finally, the DNeasy Mini spin column was placed in a clean 1.5 ml or 2 ml microcentrifuge tube and 200 µl Buffer AE was added directly onto the DNeasy membrane. The tubes were incubated at room temperature for 1 min, and then centrifuged for 1 min at 8000 rpm to elute. The PCR reactions were carried out in 0.1 ml micro centrifuge tubes. The contents for the reaction mixture were 2.5 µl Thermopol buffer, 0.5 µl each dNTPs, 1.25 µl of each forward and reverse primer, 0.2 µl Taq polymerase and 17.3 µl ultrapure nuclease free water providing total volume of 23 µl. The contents were mixed on ice in the laminar flow. In the PCR tubes, 2 μl of DNA template as added with 23 µl reaction mixture for a total reaction volume of 25 μl. The tubes were placed in thermal cycler following generic cycling parameters; initial denaturation at 95°C for 10:00 min; denaturation at 94°C for 1:00 min; annealing at 50°C for 1:00 min; elongation at 72°C for 2:00 min; cycling to step 2 for 39 more times; and followed by final elongation at 72°C for 10:00 min. The primers used in PCR were forward ITS-F (5’-TTGAACCGGGTAAAAGTCG-3’) and reverse ITS- R (5’-TTAGTTTCTTTTCCTCCGC-3’). 5 μl of the PCR product was visualized on ethidium bromide stained agarose gel in gel electrophoresis for all the strains and the presence or absence of DNA was demonstrated by the DNA bands on the gel. The strains showing smeared bands were purified using gel extraction protocol by using IBI Gel PCR DNA Fragment Extraction Kit. The purified PCR products were then sent for sequencing on both directions together. Another set of primers was used i.e. FNem18S (5’-TTGATTACGTCCCTGCCCTTT-3’) and rDNA1.58s(rev) (5’-ACGAGCCGAGTGATCCACCG-3’). The samples has been sent for sequencing in Dr. Byron Adams laboratory at Brigham Young University, Provo.
-
To test the efficacy of found native EPNs against wireworms
Nematode rearing and dose preparations: The same procedure used for rearing of available EPNs in objective 1 was followed for the rearing of native EPNs. After rearing, 4 doses; 5600, 2800, 1400, 700 IJs were prepared by counting the number of infective juveniles.
Laboratory bioassay: 500 ml plastic cups were filled with 150 g autoclaved sandy loam soil (area-140 cm2). 5 medium sized wireworm larvae were added in each cup. Once the wireworm larvae entered the soil in the cups, 4 EPN doses; 3500 IJs/cup (700 IJs/ml), 7000 IJs/cup (1400 IJs/ml), 14000 IJs/cup (2800 IJs/ml), and 28000 IJs/cup (5600 IJs/ml)) in 5 ml of water. The control cups received 5 ml of water only without any nematodes. The moisture content of soil was standardized to 15%. The wireworm mortality was observed at weekly intervals. The penetration rate was also observed whenever possible.
Statistical Analysis: Factorial ANOVA was performed to determine the effects of EPN strains, doses, and time on wireworm mortality. One way ANOVA was used to analyze the penetration of all the EPN strains. Factorial ANOVA was used to analyze the effect of promising EPN strains on the wireworm morality in shade house experiments. There was no mortality found in control so the data regarding control has not been included in the analysis. Factorial ANOVA was used to analyze the data regarding native EPN bioassay. Data from two laboratory trials repeated in time were combined as no significant interactions were detected between trials. Tukey’s HSD test was used as a post hoc test for multiple comparisons between the means at probability (α = 0.05). The data was analyzed using the software statistical package R 2.15.1 (R Development Core Team, 2017).
Results and discussion
-
Efficacy of available EPN strains against wireworms:
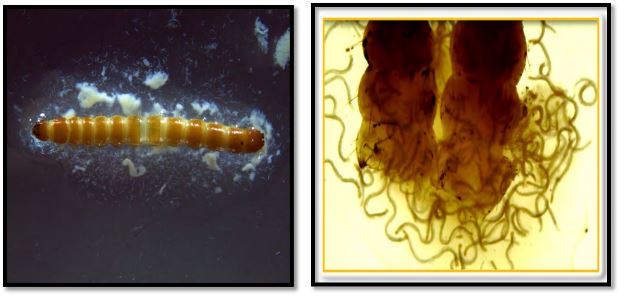
Laboratory bioassay: The data for wireworm mortality for 10 wireworms for each dose and each EPN strain was averaged for one repetition. So, overall there were 5 replications (repetitions considered as replications) with average of 10 wireworm larvae per replication. The cumulative average larval mortality at weekly intervals was calculated by taking average of 5 replications at 7 DAT (days after treatment), 14 DAT, 21 DAT, 28 DAT and statistical analysis was performed. The L. californicus larvae infected with different EPN strains are shown in figure 3.
The overall model included the effect of individual effect of EPN strain, dose, and time and interaction between these 3 factors. There was no significant interaction between EPN strain, dose and time (F = 0.263; df = 81, 640; p-value = 1.00). So, the interaction effect was removed from the model. The different EPN strains (F = 59.93; df = 9, 640; p-value <0.00001), time (F = 88.74; df = 3, 640; p-value < 0.00001), and dose (F = 40.61, df = 3, 640, p-value<0.0001) had significant effect on the larval mortality in laboratory bioassay. Also, interaction between EPN strains and doses (F = 2.532; df = 27, 640; p-value<0.0001) and between EPN strains and time (F = 2.859, df= 27, 640; p-value<0.0001) was found to be significantly different.
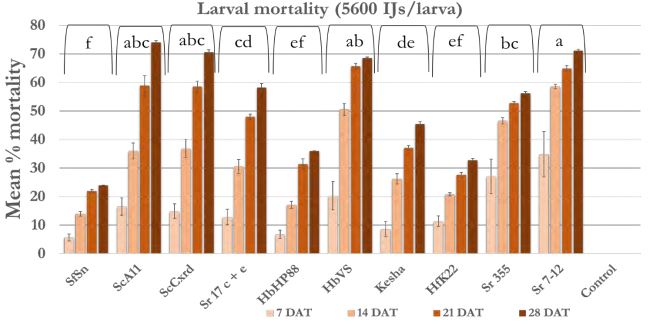
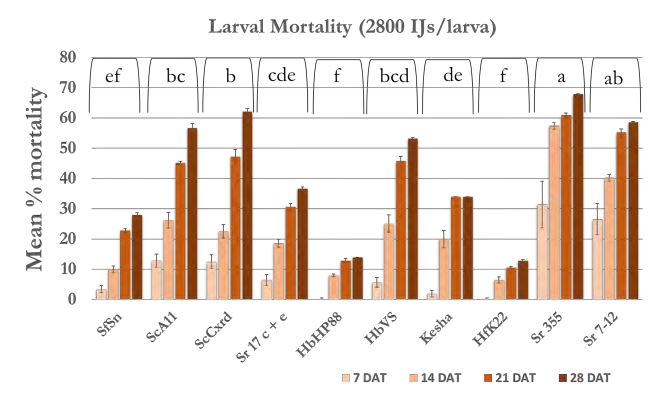
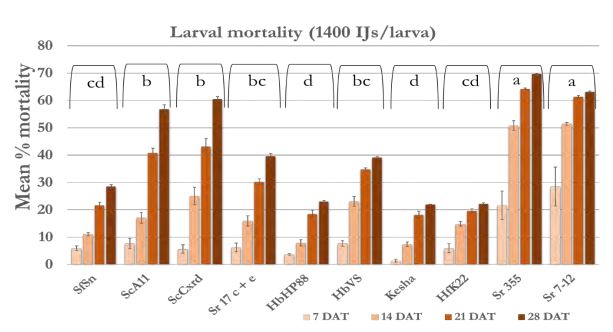
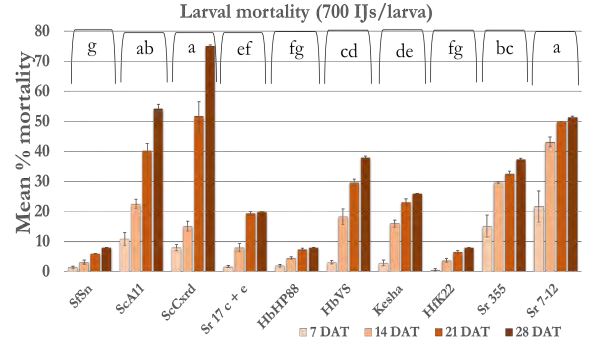
In case of 5600 IJs/larva dose (Figure 4a), the larval mortality was less than 20% after one week except Sr 355 and Sr 7-12 with 25-35% mortality. After 2 weeks, mortality reached 40% in all strains except Sr 355, Sr 7-12 and HbVS which showed 50-60% mortality. After four weeks, mortality increased and reached 70% in case of Sr 7-12, ScA11, and ScCxrd. Overall, six strains; Sr 7-12, ScA11, ScCxrd, Sr 17 c+e, Sr 355, and HbVS caused significantly higher mortality as compared to other strains. The mortality decreased as the dose decreased. In case of 2800 IJs/larva (Figure 4b), the same six strains showed high mortality (37-68%) as compared to other 4 strains. There were no significant differences between doses 2800 IJs/larva and 1400 IJs/larva with respect to wireworm larval mortality. The mortality ranged from 40-70% in the effective six strains; Sr 7- 12, ScA11, ScCxrd, Sr 355, Sr 17 c+e and HbVS in case of 1400 IJS/larva dose (Figure 4c). However, in case of 2800 IJs/larva dose, the overall mortality was higher (7-57%) as compared to 7-51% in case of 1400 IJs/larva after two weeks. In case of 700 IJs/larva dose (Figure 4d), the mortality was the lowest in all the strains except ScCxrd strain which showed 75% mortality after 4 weeks even higher than 5600 IJs/larva dose with 70% mortality. In all other strains, mortality did not exceed 40% after 4 weeks except ScA11 with 54% and Sr 7-12 with almost 51% mortality in case of 700 IJs/larva dose. Overall, strains ScA11, ScCxrd, Sr 355, Sr 7-12, Sr 17 c+e, and HbVS showed more than 50% mortality in highest dose i.e. 5600 IJs/larva dose. These promising strains and two doses, 2800 IJs/larva and 700 IJs/larva were selected for the further shade house experiments. There was no mortality found in control treatment.
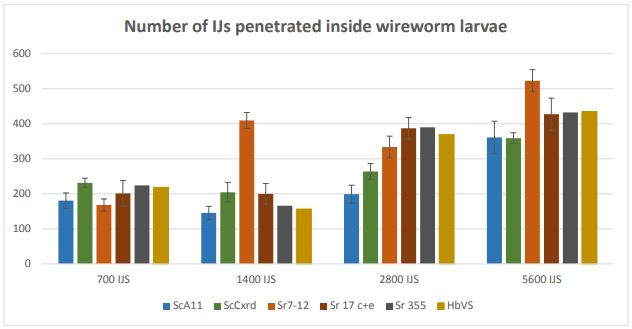
Penetration rate: The penetration rate was counted for all 10 EPN strains but average data (n=20) for effective six strains; ScA11, ScCxrd, Sr 17 c+e, Sr 355, Sr 7-12, and HbVs are presented here in Figure 5.
Overall, number of infective juveniles penetrated inside the larval bodies was higher in 5600 IJs/larva in all the strains. There were significant differences between different doses regarding the number of IJs penetrated inside the larvae except doses 700 IJs and 1400 IJs. In case of 5600 IJs, strain Sr 7-12 had more number of IJs (522.95 ± 30.89) penetrated inside the wireworm larva followed by HbVS (435.15 ± 41.18), Sr 355 (432.15 ± 52.66), Sr 17c+e (427.55 ± 45.74), ScA11 (361.15 ± 36.61), and ScCxrd (358.9 ± 29.44). In dose 1400 IJs, Sr 7-12 had highest penetration rate as compared to other strains.
Overall, these results showed that higher the dose, higher the penetration rate. It is directly correlated to the higher mortality. The reason behind the higher mortality in high doses; i.e. 5600 IJs/larva can be the higher number of IJs entering inside the larval bodies. More number of IJs might have killed the larvae faster than the lower number of IJs in the lower doses.
Shade house experiment:
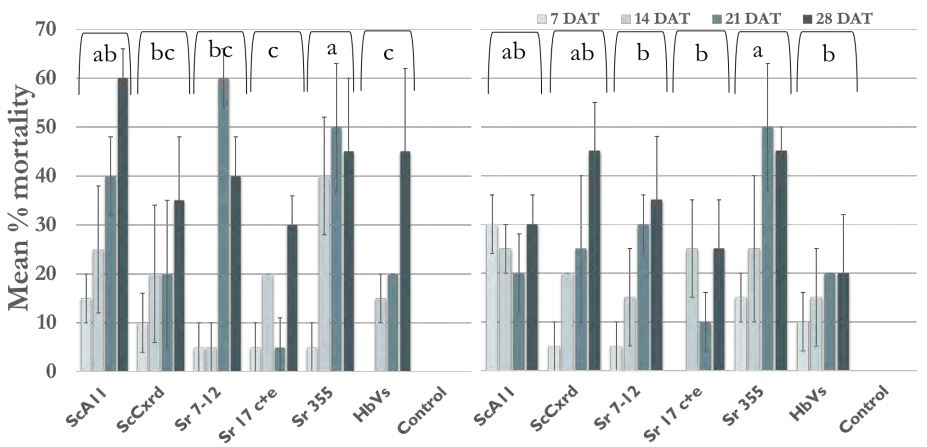
The model included the effect of individual effect of EPN strain, dose, and time and interaction between these factors. There was no significant interaction between EPN strain, dose and time (F= 1.061; df = 15, 144; p-value = 0.40). So, the interaction effect was removed from the model. The different EPN strains (F = 4.98; df = 5, 182; p-value = 0.0003) and time (F = 21.39; df = 3, 182; p-value < 0.0001) had significant effect on the larval mortality. However, 2 doses used did not have significant differences ((F = 1.18; df = 1, 182; p-value = 0.0003). All 6 strains used caused less than 30 % wireworm mortality after 2 weeks (Figure 6). But after two weeks, mortality was high all the strains. The EPN strain; Sr 355 caused significantly high mortality (40-50%) within 2- 3 weeks in both the doses. After 3 weeks, Sr 7-12 showed 60% mortality in case of 60,000 IJs but showed 30 % in case of 15,000 IJs. Similarly, ScA11 showed 60% mortality after 4 weeks in case of 60,000 IJs as compared to only 30% mortality in case of 15,000 IJs. The strain ScCxrd caused 20 to 35% mortality in case of 60,000 IJs dose and 20-45% mortality at 15,000 IJs dose. Overall, Sr 355, Sr 7-12, ScA11, and ScCxrd showed higher mortality as compared to other two strains; HbVS and Sr 17 c+e with 20-30% mortality only. There was no mortality found in control treatments.
-
To extract and identify the native EPN strains in Golden Triangle Area of Montana Overall, 19 samples out of 150 samples were found to have nematodes. But, we were able to culture only 4 samples. This might be due to a number of factors like Galleria as inappropriate host for some particular species, unfavorable environmental conditions in the laboratory, low number of nematodes present in the soil samples to infect the host. The four nematode species were named as JMS3, KP, MLS1, and MLS2 and identified using morphological and molecular techniques. The species named JMS3, KP, and MLS1 were found to be Steinernematids and MLS2 as Heterorhabditid on the basis of change in color of waxmoth larvae after nematode infection. The color was pale yellowish to grey in case of Steinernematids and reddish brown for Heterorhabditid infected larvae. The measurements regarding morphological observations are given in Tables 2, 3, 4, and 5. We are still waiting on the sequencing results from Dr. Byron Adams.
-
To test the efficacy of found native EPN strains against wireworm
The different EPN strains were significantly different in regards to larval mortality (F = 26.64; df= 5, 668; p-value <0.00001). The other factors; time (F = 51.29; df = 3, 668; p-value < 0.00001), and dose (F = 22.98, df = 3, 668, p-value < 0.00001) had significant effect also on the larval mortality . Overall, JMS3 showed significantly higher mortality followed by KP, MLS1, and MLS2.
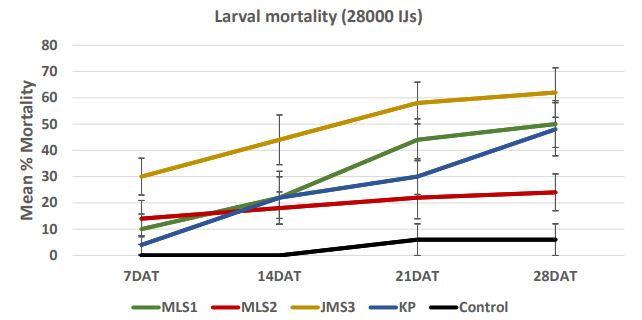
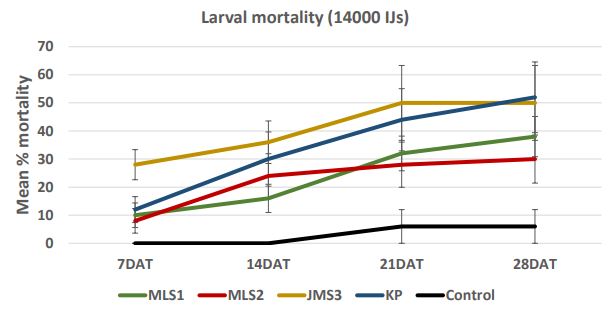
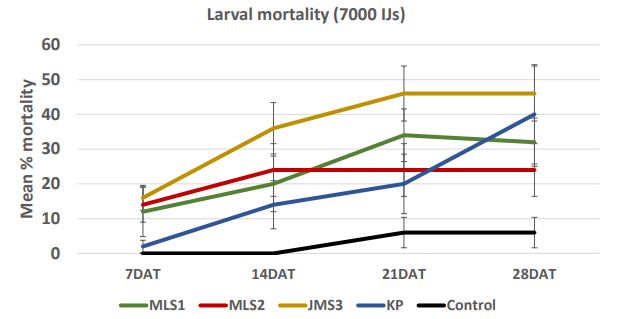
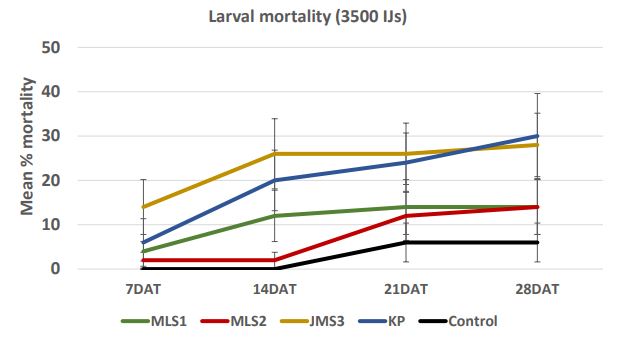
In case of 28000 IJs dose (Figure 7a), the larval mortality was less than 30% after one week. After 2 weeks, the mortality reached 58% in JMS3 strain. After four weeks, mortality increased and reached more than 50% in case of JMS3 (62%) and MLS1 (50%). The range of mortality decreased as the dose was low. In case of 14000 IJs (Figure 7b), the strains KP caused higher mortality (12- 52%) followed by JMS3 with 28-50% mortality in 4 weeks. There were no significant differences between two high doses; 28000 IJs and 14000 IJs with respect to wireworm larval mortality. The mortality was lower in dose 7000 IJs and did not exceed 50% (Figure 7c) even in JMS3 and KP. In case of 3500 IJs dose (Figure 7d), the mortality was the lowest in all the strains and did not exceed 30 %. The mortality remained same after 2 weeks in JMS3 and KP and the mortality was almost double as compared to other 2 strains i.e. MLS1 and MLS2. Overall, JMS3 and KP were found to be effective against wireworms and will be used in further experiments. The mortality remained almost same after 3 weeks as there was no significant difference between mortality at 3 weeks and mortality at 4 weeks after nematode treatments.
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank, Jonathan Blanchard and Harold Miller for their assistance. Also, we would like to thank Dr. Adams Byron for letting us use his laboratory facilities for the molecular identification of native EPNs.
References
Adams, B. J., Peat, S. M., and Dillman, A. R. (2007) Phylogeny and evolution. In Entomopathogenic Nematodes: Systematics, Phylogeny, and Bacterial Symbionts; Nguyen, K.B., Hunt, D.J., Eds.; Brill: Boston, MA, USA, Volume 5, pp. 693–733.
Bedding, R. A. and R. J. Akhurst. (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 21, 109–116.
Kaya, H. K. and Stock, S. P. (1997) Techniques in insect Nematology. In: Lacey LA (ed) Manual of Techniques in Insect Pathology, pp 281±324. Academic Press, New York.
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available: http://www.R-project.org. Cited 19 December 2017.
Figure 3. Nematodes infected wireworm larvae; Left: IJs emerging out of the wireworm larva, Right: EPN adults after dissecting the dead wireworm larva
Figure 4a. Mean percentage larval mortality at 5600 IJs/larva
Figure 4b. Mean percentage larval mortality at 2800 IJs/larva
Figure 4c. Mean percentage larval mortality at 1400 IJs/larva
Figure 4d. Mean percentage larval mortality at 700 IJs/larva
Figure 5. Number of infective juveniles penetrated inside wireworm larvae
Figure 6. Percentage mean larval mortality; Left: larval mortality at 60000 IJs/pot; Right: larval mortality at 15000 IJs/pot
Table 2. Morphology of JMS3
|
Character |
1st gen female |
1st gen male |
2nd gen female |
2nd gen male |
IJ |
|
Body length (L) |
4541.9 ± 376.51 (2137- 7291) |
1494 ± 57.18 (1128-2086) |
1857.25 ± 52.09 (1543- 2491) |
997.3 ± 45.15 (724-1578) |
663.05 ± 9.05 (618-793) |
|
Maximum Body dia (D) |
198.2 ± 8.19 (150-281) |
127.35 ± 4.63 (92-163) |
115 ± 3.78 (85-163) |
80.35 ± 2.05 (66-100) |
25.86 ± 0.56 (21.2-30.3) |
|
Stoma length |
6.6 ± 0.33 (4-9) |
|
5.5 ± 0.26 (4-8) |
|
|
|
Stoma width |
6.7 ± 0.27 (4-9) |
|
7.25 ± 0.25 (6-9) |
|
|
|
EP |
96.5 ± 2.02 (82-118) |
98.05 ± 2.22 (76-114) |
82.85 ± 2.00 (69-110) |
78.85 ± 2.23 (61-94) |
59.38 ± 0.83 (53-69) |
|
NR |
119.65 ± 3.79 (101-156) |
105.6 ± 2.18 (89-123) |
109.05 ± 3.38 (91-163) |
100.435 ± 2.09 (75-114) |
65 ± 0.84 (57-72) |
|
ES |
188.95 ± 3.99 (161-218) |
152.4 ± 1.85 (133-171) |
165.4 ± 2.16 (150-191) |
143.05 ± 4.24 (113-199) |
91.45 ± 0.78 (84-98) |
|
Tail length with sheath (T) |
61.35 ± 2.18 (45-78) |
46.05 ± 1.36 (32-59) |
50.1 ± 1.63 (38-64) |
41.7 ± 1.09 (35-51) |
57.43 ± 1.16 (41-64.1) |
|
Tail length without sheath |
|
|
|
|
36.13 ± 1.00 (25.2-43) |
|
Anal body diameter (ABD) |
88.95 ± 5.01 (60-125) |
59.65 ± 1.89 (39-75) |
55.25 ± 2.02 (42-69) |
51 ± 1.17 (42-62) |
|
|
Distance from anterior end to vulva |
2006.2 ± 128.008 (1236- 3218) |
|
1055.4 ± 50.59 (768-1423) |
|
|
|
Spicule length (SP) |
|
75.25 ± 1.64 (54-89) |
|
68.15 ± 1.99 (51-82) |
|
|
Spicule width |
|
10.55 ± 2.18 (54-89) |
|
11.2 ± 0.47 (8-16) |
|
|
Gubernaculum length (GU) |
|
51.1 ± 1.42 (39-61) |
|
41.7 ± 0.85 (33-49) |
|
|
V |
48.87 ± 4.20 (28.40-96.09) |
|
57.57 ± 3.12 (35.12-88.59) |
|
|
|
A |
|
|
|
|
25.89 ± 0.66 (21-30.75) |
|
B |
|
|
|
|
7.26 ± 0.11 (6.61-8.62) |
|
C |
|
|
|
|
11.65 ± 0.31 (9.64 ± 15.61) |
|
D% = EP/ES × 100 |
|
64.42 ± 1.44 (53.80-75.34) |
|
79.14 ± 2.61 (53.51- 96.91) |
64.98 ± 0.92 (57.77- 71.43) |
|
E% = EP/T × 100 |
|
|
|
|
104.38 ± 2.87 (89.37-145.61) |
|
SW% = SP/ABD × 100 |
|
127.96 ± 3.79 (105.33-168.89) |
|
134.83 ± 4.90 (98.08- 185.71) |
|
|
GS% = GU/SP ×100 |
|
68.57 ± 2.42 (48.15-89.71) |
|
62.01 ± 1.90 (50.77- 85.19) |
|
Table 3. Morphology of KP
|
Character |
1st gen female |
1st gen male |
2nd gen female |
2nd gen male |
IJ |
|
Body length (L) |
2697.95 ± 195.88 (1723- 4942) |
1570.35 ± 36.84 (1259-1813) |
2205.65 ± 47.34 (1836-2546) |
1189.4 ± 24.85 (980-1464) |
820.25 ± 11.16 (711-900) |
|
Maximum Body dia (D) |
140.85 ± 4.71 (119-207) |
96.93 ± 3.01 (76-130) |
92.5 ± 2.88 (73-129) |
86.32 ± 2.65 (65-119) |
28.48 ± 0.73 (22-34.4) |
|
Stoma length |
8.1 ± 0.43 (6-15) |
|
7.4 ± 0.40 (5-11) |
|
|
|
Stoma width |
10.95 ± 0.60 (6-16) |
|
10.15 ± 0.70 (6-20) |
|
|
|
EP |
84.75 ± 2.52 (72-106) |
90.06 ± 2.25 (73-115) |
100.15 ± 3.4 (59-124) |
69.65 ± 1.023 (60-78) |
64.45 ± 1.94 (48-80) |
|
NR |
110.3 ± 3.16 (91-141) |
98.35 ± 1.63 (88-113) |
98.95 ± 2.51 (69-115) |
93.6 ± 1.35 (82-103) |
72 ± 2.83 (42-91) |
|
ES |
165.85 ± 3.49 (134-193) |
145.55 ± 2.80 (121-167) |
144.25 ± 4.02 (95-164) |
135.2 ± 1.94 (110-149) |
90.2 ± 2.97 (61-114) |
|
Tail length with sheath (T) |
67.7 ± 3.15 (48-85) |
41.65 ± 0.75 (36-48) |
50.2 ± 1.72 (34-67) |
47.35 ± 0.61 (41-52) |
79.34 ± 1.49 (61-90) |
|
Tail length without sheath |
|
|
|
|
52.66 ± 1.11 (42-60) |
|
Anal body diameter (ABD) |
44.35 ± 2.45 (28-73) |
44.72 ± 1.07 (35-51) |
40.15 ± 1.88 (26-58) |
45.5 ± 1.20 (38-56) |
|
|
Distance from anterior end to vulva |
1425.85 ± 61.1 (970-2000) |
|
1188.15 ± 31.83 (1021-1690) |
|
|
|
Spicule length (SP) |
|
80.35 ± 1.41 (67-92) |
|
69.85 ± 0.93 (62-77) |
|
|
Spicule width |
|
9.4 ± 0.56 (6-14) |
|
10 ± 0.41 (6-13) |
|
|
Gubernaculum length (GU) |
|
49.95 ± 1.11 (41-59) |
|
49.4 ± 1.03 (40-56) |
|
|
V |
58.05 ± 4.42 (23.48-93.44) |
|
54.52 ± 2.20 (40.55-89.42) |
|
|
|
A |
|
|
|
|
29.15 ± 0.81 (24.58-39.68) |
|
B |
|
|
|
|
9.31 ± 0.36 (7.45-13.15) |
|
C |
|
|
|
|
10.40 ± 0.21 (8.67-13.14) |
|
D% = EP/ES × 100 |
|
62.09 ± 1.47 (49.08-72.34) |
|
51.72 ± 1.05 (45.45-62.5) |
72.42 ± 2.36 (55.24-87.91) |
|
E% = EP/T × 100 |
|
|
|
|
81.60 ± 2.63 (63.16-108.11) |
|
SW% = SP/ABD × 100 |
|
181.27 ±4.51 (145.65- 235.90) |
|
156.033 ± 5.18 (114.29) |
|
|
GS% = GU/SP ×100 |
|
62.74 ± 2.13 (49.40-85.51) |
|
71.11 ± 1.97 (53.25-83.08) |
|
Table 4. Morphology of MLS1
|
Character |
1st gen female |
1st gen male |
2nd gen female |
2nd gen male |
IJ |
|
Body length (L) |
2636.2 ± 87.81 (2013-3800) |
1304.8 ± 43.3 (913-1570) |
1368.85 ± 32.62 (1089-1672) |
1442.5 ± 43.20 (1117- 1940) |
807.85 ± 9.60 (743-906) |
|
Maximum Body dia (D) |
157.9 ± 3.51 (112-178) |
96.05 ± 3.14 (65-119) |
83.74 ± 1.83 (62-93) |
94.1 ± 3.50 (62-137) |
29.93 ± 0.57 (26-35) |
|
Stoma length |
18.6 ± 0.91 (12-27) |
|
6.79 ± 0.31 (4-9) |
|
|
|
Stoma width |
17.9 ± 0.87 (10-25) |
|
10.25 ± 0.53 (7-18) |
|
|
|
EP |
115.7 ± 5.34 (79-160) |
93 ± 1.55 (84-109) |
89.15 ± 2.46 (66-103) |
93.1 ± 1.29 (83-107) |
55.25 ± 1.72 (43-67) |
|
NR |
140.45 ± 5.57 (89-201) |
100 ± 1.18 (88-110) |
115.1 ± 1.87 (101-131) |
109.05 ± 1.52 (98-120) |
59.65 ± 2.01 (48-81) |
|
ES |
179.25 ± 3.20 (160-212) |
164.55 ± 1.72 (154-180) |
175.65 ± 1.69 (160-185) |
155.95 ± 1.50 (143-168) |
87.7 ± 2.02 (48-81) |
|
Tail length with sheath (T) |
71.25 ± 3.00 (55-114) |
52.75 ± 2.62 (34-72) |
58.4 ± 1.67 (44-78) |
64.75 ± 1.57 (53-82) |
80.85 ± 1.09 (74-89) |
|
Tail length without sheath |
|
|
|
|
26.5 ± 0.55 (22-31) |
|
Anal body diameter (ABD) |
93.75 ± 2.50 (77-117) |
62.85 ± 3.00 (45-91) |
42.55 ± 1.00 (35-49) |
59.75 ± 1.64 (48-73) |
|
|
Distance from anterior end to vulva |
1239.6 ± 30.58 (1044-1540) |
|
670.75 ± 12.13 (557-760) |
|
|
|
Spicule length (SP) |
|
71.5 ± 2.21 (58-98) |
|
75.65 ± 1.96 (63-91) |
|
|
Spicule width |
|
9.9 ± 0.58 (5-15) |
|
7.8 ± 0.70 (2-13) |
|
|
Gubernaculum length (GU) |
|
45.85 ± 0.86 (40-53) |
|
50.52 ± 1.45 (43-66) |
|
|
V |
47.022 ± 1.41 (36.29-59.26) |
|
49.42 ± 1.25 (38.84-64.92) |
|
|
|
A |
|
|
|
|
27.19 ± 0.6 (23.23-32.78) |
|
B |
|
|
|
|
9.31 ±0.25 (7.40-11.13) |
|
C |
|
|
|
|
10.02 ± 0.16 (8.78-11.19) |
|
D% = EP/ES × 100 |
|
56.56 ± 0.86 (51.14-65.19) |
|
59.83 ± 1.09 (52.17-72.29) |
63.62 ± 2.37 (39.81-83.33) |
|
E% = EP/T × 100 |
|
|
|
|
68.42 ± 2.11 (51.81-86.84) |
|
SW% = SP/ABD × 100 |
|
117.34 ± 6.81 (63.74-168) |
|
127.94 ± 3.88 (91.30- 163.46) |
|
|
GS% = GU/SP ×100 |
|
66.35 ± 2.16 (51.19-91.38) |
|
67.36 ± 2.09 (48.86-90.47) |
|
Table 5. Morphology of MLS2
|
Character |
Hermaphrodite |
2nd gen male |
2nd gen female |
IJ |
|
Body length (L) |
3182.5 ± 121.15 (2260-4300) |
802.1 ± 20.16 (610-920) |
1647.35 ± 85.89 (1100-2740) |
602.25 ± 9.48 (513-654) |
|
Maximum Body dia (D) |
179.15 ± 4.07 (147-213) |
62.3 ± 1.99 (50-78) |
117 ± 3.49 (92-150) |
24.74 ± 0.64 (18-31) |
|
Stoma length |
8.25 ± 0.38 (6-11) |
|
6.27 ± 0.26 (4-8) |
|
|
Stoma width |
9.85 ± 0.52 (6-13) |
|
9.65 ± 0.38 (7.5-13) |
|
|
EP |
147.15 ± 1.85 (138-168) |
77.9 ± 1.17 (70-90) |
92.25 ± 1.42 (79-104) |
77.35 ± 2.24 (55-95) |
|
NR |
159.5 ± 3.44 (148-216) |
80.45 ± 1.32 (70-91) |
89.55 ± 2.16 (72-108) |
78.2 ± 1.32 (68-90) |
|
ES |
249.25 ± 8.90 (202-327) |
110.25 ± 1.97 (97-129) |
126.85 ± 1.77 (109-141) |
106.5 ± 3.10 (93-140) |
|
Tail length with sheath (T) |
111.55 ± 2.20 (98-125) |
61.58 ± 3.59 (41-110) |
64.2 ± 2.77 (46-90) |
91.26 ± 4.52 (56-140.3) |
|
Tail length without sheath |
|
|
|
57.09 ± 4.17 (37-101) |
|
Anal body diameter (ABD) |
54.7 ± 1.38 (43-65) |
34.51 ± 0.67 (30-39.4) |
64.35 ±3.36 (41-91) |
|
|
Distance from anterior end to vulva |
1404 ± 33.52 (1080-1650) |
|
862.5 ± 38.06 (610-1290) |
|
|
Spicule length (SP) |
|
41 ± 1.26 (32-51) |
|
|
|
Spicule width |
|
3.6 ± 0.27 (2-6) |
|
|
|
Gubernaculum length (GU) |
|
22.95 ± 0.74 (14-28) |
|
|
|
V |
45.15 ± 1.81 (35.12-60.25) |
|
55.35 ± 4.49 (32.34-117.27) |
|
|
A |
|
|
|
24.34 ± 0.60 (14.93-28.5) |
|
B |
|
|
|
5.65 ± 0.18 (3.063-5.52) |
|
C |
|
|
|
6.60 ± 0.29 (2.10-9.16) |
|
D% = EP/ES × 100 |
|
70.90 ± 1.19 (63.2-83.50) |
|
72.63 ± 2.61 (72.29-59.14) |
|
E% = EP/T × 100 |
|
|
|
84.75 ± 5.04 (49.52-98.21) |
|
SW% = SP/ABD × 100 |
|
119.83 ± 4.69 (91.37-164.52) |
|
|
|
GS% = GU/SP ×100 |
|
56.85 ± 2.61 (41.18-87.5) |
|
|
Figure 7a. Mean percentage larval mortality at 28000 IJs/cup
Figure 7b. Mean percentage larval mortality at 14000 IJs/cup
Figure 7c. Mean percentage larval mortality at 7000 IJs/cup
Figure 7d. Mean percentage larval mortality at 7000 IJs/cup