Evaluation of the effectiveness of entomopathogenic fungus and trap crops for the management of wireworms on spring wheat
Principal Investigators: Dr. Michael A. Ivie1 , Dr. Gadi V.P. Reddy2,
Project collaborators: Stefan T. Jaronski3, Kevin Wanner2 and Heikki M. Hokkanen4, Dr. David I Shapiro Ilan5, Dr. Byron Adams6, Dr. Vincent H. Smith7
Project personnel: Anamika Sharma8, Ramandeep Kaur Sandhi1, Ramadevi Gadi8
- Department of Plant Science and Plant Pathology, Montana State University, Bozeman,
- USDA-ARS-Southern Insect Management Research Unit, 141 Experiment Station Rd., P.O. Box 346, Stoneville, MS 38776, USA
- United States Department of Agriculture, Agricultural Research Service, Northern Plains Agricultural Research Laboratory, 1500 N. Central Avenue, Sidney, MT-Retired
- Department of Agricultural Sciences, University of Helsinki, FI-00014 Helsinki, Finland
- Fruit and Tree Nut Research, USDA-ARS, Georgia.
- Brigham Young University, Provo, Utah.
- Department of Agricultural Economics and Director of the Agricultural Marketing Policy Center at Montana State University.
- Western Triangle Agricultural Research Center, Montana State University, Conrad, MT
Aim of the study
The aims of this study were: 1) to evaluate the effectiveness of trap crops for the management of wireworms and 2) to evaluate efficacy of entomopathogenic fungi for wireworm management under lab and field conditions.
Figure 1: Wireworms feeding on spring wheat and pupa of wireworm in the soil.
Material and Methods Study sites
In 2019, two sites were selected for evaluating entomopathogenic fungus. Both sites were pre analyzed for presence of wireworms before commencing the experiments. One site was irrigated (Choteau; N47o 90.238’ W112o 23.802’) and one site was non-irrigated (Pendroy; N48o 56.009’ W111o 40.565’). The wireworm pressure was moderate to high in the selected sites.
Experimental design
In 2017 and 2018, different fungal strains on nutritive carriers (polenta, millet, and couscous) were tested in the field conditions. In 2019, the selected high performing EPFs were tested based on 2017 and 2018 results (Sharma et al. 2019). In 2019, these EPFs were applied in the selected fields with conventional farming. The non-irrigated Pendroy site had barley grown and irrigated Choteau site had spring wheat. In both fields, we asked growers to follow conventional farming practice. After the seeds were sown, next day the EPFs were applied in the furrows along the seeds with the help of a harrow (at the rate of 5gms/plot Table 1). The fungus formulations were prepared by USDA ARS, Sidney MT.
The experiment used a Randomized Complete Block Design (for EPF experiment) with a total of 36 plots [including four replications. Each plot was 4×4 m. Buffer zones of 1.5 m were maintained between plots. The non-irrigated field (Pendroy) barley (Hockett) was shown on 9 May 2019 @20 seeds/ft2 with a spacing of 10 inches. Dry fertilizers with drill were applied. Five gallons/acre nitrogen was applied. No pre-emergence herbicides were applied. Before seeding Roundup was applied (16-20 ounces). Seeds were treated with imidacloprid (Gaucho® 600, Bayer Crop Science). In the irrigated field (Choteau) spring wheat was seeded (Clear field) at 7.5 inches spacing on 10 May 2019. Fertilizers 15 gallons of ‘thirty two’ nitrogen, 20-10-5-10 (140 gallons/acre) and 25 tonn of manure (120 pounds/acre) were applied. Beyond and Wildcard were applied at label rates as herbicides. Non-irrigated sites did not receive any water. Irrigated sites received 5 cm of water via overhead irrigation once a week. The treatments were, millet, couscous, Beauveria bassiana GHA millet, Beauveria bassiana GHA couscous, Beauveria bassiana ERL836 millet, Beauveria bassiana ERL836 couscous, Metarhizium robertsii DWR2009 millet, and Metarhizium robertsii DWR2009 couscous. All EPF treatments were applied with seeds in furrows (at the rate of 5gms/plot Table 1). The fields were seeded at Pendroy (14 May and 16 May), Choteau and Valier (24 May) and Ledger (29 May 2018). The first irrigation took place within 30 days of planting in irrigated fields. The irrigated field was harvested on 29 August 2019 and the non-irrigated field was harvested on 12 September 2019.
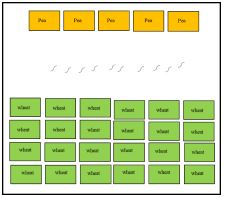
For the trap crop experiment, in 2019 green house experiment was setup based on the results generated in 2017 (Sharma et al. 2017). Six plastic containers (60cmx50cmx50cm) were established in green-house conditions. In every container sandy loam soil from WTARC fields was filled. Once the soil was moisturized first the chickpea seeds ‘Orion’ variety. After 10 days spring wheat ‘Duclair’ variety were seeded. The chickpeas were planted on one side of the container (5 seeds) and spring wheat (25 seeds) were planted on another side of the container (Figure 2). After, 7 days 10 wireworms (Limonius californicus) were released in the middle of the containers. One container was kept as a control container where no wireworms were released. After every 7 days plant damage was recorded. In 45 days the experiment was dismantled by de- rooting the plants and the number of wireworms were recorded. The number of wireworms associated with chickpeas and spring wheat was reported. The experiment was established two times. The first experiment was established on 14th June 2019 and the second was established on 22nd July 2019.
Figure 2: The schematic diagram of the trap crop experiment, where spring wheat seeds and chickpea seeds were planted to access the attractiveness of the chickpeas to wireworms.
Sampling for plant damage
To determine the level of crop damage from wireworms, the number of seedlings in each plot was measured randomly using the 1 m line intercept method. Two counts were taken from each plot, from the two middle rows. The first count was taken two weeks after planting. The starting and ending points of the sample areas (n = 2; each 1 m in length) were labeled with wooden stakes, so that the same group of seedlings could be recounted each time. Subsequent counts were taken at two-week intervals at both sites. At harvest, the height of these same marked plants was recorded using a wooden meter scale (Washington, USA).
Sampling for wireworm density
To determine the density of wireworm larvae, traps were established in each plot areas following the soil sampling bait-trap method of Reddy et al. (2014), which consisted of stockings filled with a mixture of wheat and barley. The traps were soaked for 24 hours to make the seeds sprout which is attractive to wireworms due to CO2 release. The traps were buried in 8–15 cm deep hole and were covered with black plastic to provide an amenable environment to wireworms. Traps were then collected at 15 days and 30 days post-deployment for assessment of larval numbers. Traps with wireworms were brought to the Western Triangle Agricultural Research Center (WTARC), Conrad, Montana. At WTARC, traps were processed in Berlese Funnels (Bioquip products, California, USA; wooden stands set up for Berlese Funnel were built at WTARC) and wireworms were separately collected from each plot and identified using keys described by Etzler (2013).
Post-harvest data collection
Before harvesting, the plot’s surface areas were calculated. After harvesting, wheat and barley from each plot were brought to the WTARC facility and cleaned using a seed cleaning machine (Almaco, Allan Machine Company, Iowa, USA). The plot and test weight was measured using a laboratory balance (Ohaus, Adventure™ Pro model AV8101). Wheat samples were processed through a grain analyzer (Perten Instruments IM9500; Hägersten, Sweden) to determine grain moisture and protein.
Statistical analysis
Analysis of variance (ANOVA) was carried out using the PROC Mixed procedure in SAS 9.4 (PROC Mixed, SAS Institute 2018). Data were pooled for each replicate, and treatments were considered as fixed effects while the block was considered a random effect. Normality of data was tested with a Univariate procedure (PROC Mixed). Estimates of least square means and differences of least square means were evaluated (Type 3 test of fixed effects F-test). Multiple comparisons among the treatments were made using Fisher’s Least Significant Test (LSD) at α = 0.05 by using the standard error generated in ANOVA.
Results
Evaluation of efficacy of entomopathogenic fungus
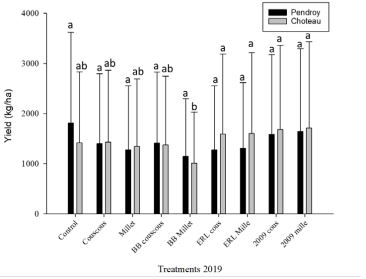
In 2019, we applied selected EPFs based on the 2017 and 2018 results (Sharma et al. 2019). These EPFs were tested in irrigated and non-irrigated fields where seed treatment (imidacloprid) was also applied to the seeds. At Pendroy site with barley yield had no significant variation with any of the nine treatments (F=1008; df=24; P=0.96). Numerically higher yields were associated with Control plots. Whereas at Choteau site (irrigated site with spring wheat) four treatments (Beauveria bassiana ERL836 Couscous, Beauveria bassiana ERL836 Millet, Metarhizium robertsii DWR2009 Couscous, Metarhizium robertsii DWR2009 Millet) performed significantly better than other treatments and control (F=437; df=24; P=0.10). In terms of plant count and test weight of seeds no significant difference was found. At Pendroy site the greater wireworm pressure resulted in no yield with some treatments (Figure 3; Table 2).
Evaluation of the effectiveness of trap crops
In the trap crop experiment, according to last results (Adhikari and Reddy 2017; Sharma et al. 2018), pulses especially peas attracted wireworms towards them. In 2018 we established a spilt plot design to access the border cropping design and found no significant difference. However in 2018, at Northwest Agriculture Experiment Station MSU (Kalispell) it was recorded that in a variety trial experiment among different chickpea varieties wireworms preferred ‘Orion’ chickpea variety and fed on the Orion plots completely. Therefore in 2019 we decided to access the attractiveness of Orion chickpea with wheat plants. In two sets of experiments, the number of wireworms found associated with chickpeas and wheat plants were equal (n=50), however more damage was found with wheat plants (58%) in comparison to the chickpea plants (42%). When we compare with control container where no wireworms were released, in control container the germination and survival of chickpea plants was 80% and wheat plants was 76% and in treated containers (10) the survival rate of chickpea plants was 58% and wheat plant was 42.4% (Table 3; Figure 4).
Conclusion
In 2017, granular formulations of three EPFs, on polenta and millet spent substrate carriers, were applied in-furrow at planting, at two rates, against a water control and imidacloprid seed treatment in spring wheat in Montana, USA. The selected EPFs were Beauveria bassiana GHA, Metarhizium robertsii DWR356, M. robertsii DWR2009, applied as granular formulations at 11 kg ha−1 or 22 kg ha−1. In 2017, at Valier, DWR356, DWR2009 on millet carrier at 22.4 kg ha−1 provided greater yield, but all the treatments at the lower rate were still cost-effective. In 2018, B. bassiana GHA and M. robertsii DWR2009 were retested along with B. bassiana ERL836 and M. brunneum F52. Millet carrier alone, GHA and ERL836 on millet carrier obtained cost-effective results at irrigated and non-irrigated sites in 2018. However, these were less cost-effective than imidacloprid as a seed treatment. Earlier it was recorded that fungus along with seed treatment (imidacloprid) provide improved protection to the wheat plants (Antwi et al. 2018). Therefore in 2019, we selected high performing EPFs based on 2017 and 2018 results and applied in the fields where seed treatment was applied to the seeds. In 2019 at the non-irrigated site with barley crop no significant difference was observed with any EPF, rather control plots had a numerically higher yield. Nevertheless, in this field due to the high pressure of wireworms (<5 wireworms per trap) and a greater population of weeds, some of the plots had zero yield. Hence less yield cannot be directly related to the efficacy of EPFs. In the irrigated field with spring wheat crop B. bassiana ERL836 on couscous and millet carrier and M. robertsii DWR2009 on couscous and millet carrier both provided greater protection to the wheat plants.
In the trap crop experiment, the experiment was done in the green house to access the attractiveness of chickpea ‘orion’ variety to wireworms. This experiment indicated some degree of attractiveness if the wheat plants and chickpea plants are in close vicinity. An equal number of wireworms were associated with both the crops but greater damage was reported to the wheat plants.
Table 1: Materials, rates, and methods of application for treatments applied in study of wireworm control at non-irrigated (Pendroy) and irrigated site (Choteau), Montana in 2019. Plot size: 4x4 meters.
|
Treatment |
Material |
Rate |
Source |
|
T1: |
Control (Water) |
|
|
|
T2: |
Couscous |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T3: |
Millet |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T4: |
Beauveria bassiana GHA Couscous |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T5: |
Beauveria bassiana GHA Millet |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T6: |
Beauveria bassiana ERL836 Couscous |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T7: |
Beauveria bassiana ERL836 Millet |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T8: |
Metarhizium robertsii DWR2009 Couscous |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
|
T9: |
Metarhizium robertsii DWR2009 Millet |
(10lb/acre) 18.5 gms/plot |
Stefan T. Jaronski USDA ARS |
Table 2: Impact of Entomopathogenic fungus treatments on spring wheat and barley performance in 2019 in terms of plant count, yield, and test weight. Standard error and least significant differences are calculated with the means generated by PROC MIXED analysis (α=0.05). The treatments were applied in Randomized Block Design (n=4); water was used as control.
|
Non-irrigated sites |
|
|
|
Irrigated sites |
|
|
|
Pendroy |
|
|
|
Choteau |
|
|
|
Treatments |
Plant Count |
Yield (kg/ha) |
Test Weight (gms) |
Plant Count |
Yield (kg/ha) |
Test Weight (gms) |
|
Control (Water) |
7.2±1.3a |
1810±547a |
0±15a |
32±2.7a |
1416±160ab |
74±8.6b |
|
Couscous |
5.5±1.3a |
1398±547a |
17±15a |
34±2.7a |
1433±160ab |
72.9±8.6b |
|
Millet |
5.3±1.3a |
1279±547a |
32±15a |
34±2.7a |
1346±160ab |
72.3±8.6b |
|
Beauveria bassiana GHA Couscous |
6±1.3a |
1412±547a |
35±15a |
34±2.7a |
1374±160ab |
71.9±8.6b |
|
Beauveria bassiana GHA Millet |
5.3±1.3a |
1150±547a |
33±15a |
33±2.7a |
1014±160b |
98.5±8.6a |
|
Beauveria bassiana ERL836 Couscous |
5.6±1.3a |
1408±523a |
40±14a |
33±2.7a |
1559±143a |
72.2±7.7b |
|
Beauveria bassiana ERL836 Millet |
5.2±1.3a |
1310±547a |
18±15a |
33±2.7a |
1606±160a |
73±8.6b |
|
Metarhizium robertsii DWR2009 Couscous |
6.1±1.3a |
1473±591a |
0±18a |
31±3.2a |
1769±185a |
72±10b |
|
Metarhizium robertsii DWR2009 Millet |
6±1.3a |
1646±547a |
0±15a |
31±2.7a |
1716±160a |
73.4±8.6b |
|
F (P) |
2.2 (0.83) |
1008 (0.96) |
42 (0.39) |
7.5 (0.99) |
437 (0.10) |
22 (0.45) |
Figure 3: Mean yield (kg/ha) of wheat at irrigated field [Choteau; ( )] and barley at non-irrigated field [Pendroy; ( )] in 2019, [n=2]. Different letters above the bars indicate significant differences (α= 0.05). y-axis shows mean yield (mean yield+ SE) and x-axis indicates nine treatments.
Figure 4: Orion chickpea damaged by wireworms.
Table 3: Performance of ‘Orion’ chickpea and ‘Duclair’ spring wheat in containers.
|
|
Container 1 Control |
Container 2 |
Container 3 |
Container 4 |
Container 5 |
Container 6 |
||||||
|
Experiment 1 |
No. of plants |
No. of wirew orms |
No. of plants |
No. of wirewo rms |
No. of plants |
No. of wirewo rms |
No. of plants |
No. of wirewor ms |
No. of plants |
No. of wirew orms |
No. of plants |
No. of wirewo rms |
|
Orion chickpea |
4 |
0 |
1 |
3 |
3 |
2 |
3 |
7 |
4 |
5 |
4 |
5 |
|
Duclair wheat |
18 |
0 |
8 |
7 |
16 |
8 |
9 |
3 |
8 |
5 |
8 |
5 |
|
Experiment 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Orion chickpea |
4 |
0 |
2 |
5 |
2 |
6 |
4 |
4 |
3 |
6 |
3 |
7 |
|
Duclair wheat |
20 |
0 |
10 |
5 |
15 |
4 |
11 |
6 |
9 |
4 |
12 |
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total chickpea |
8 |
0 |
3 |
8 |
5 |
8 |
7 |
11 |
7 |
11 |
7 |
12 |
|
Total spring wheat |
38 |
0 |
18 |
12 |
31 |
12 |
20 |
9 |
17 |
9 |
20 |
8 |
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank Shad Chrisman, Julie Prewett and summer intern Carley Ries for assistance with field work. We would also like to thank the producers, Jonathan Stoltz and Mike Leys for providing us the field to establish the experiments.
References
Antwi FB, Shrestha G, Reddy GVP, Jaronski ST (2018) Entomopathogens in conjunction with imidacloprid could be used to manage wireworms (Coleoptera: Elateridae) on spring wheat. Canadian Entomologist 150,124–139.
Adhikari A, and Reddy GVP. 2017. Evaluation of trap crops for the management of wireworms in spring wheat in Montana. Arthropod Plant Interact. 11, 755–766. DOI 10.1007/s11829-017- 9533-5.
Etzler FE. 2013. Identification of economic wireworms using traditional and molecular methods.
M.S. thesis dissertation, Montana State University, Bozeman, Montana.
Reddy, GVP, Tangtrakulwanich K, Wu, Miller JH, Ophus VL, Prewett J, and Jaronski ST. 2014. Evaluation of the effectiveness of the entomopathogens for the management of wireworms (Coleoptera: Elateridae) on spring wheat. J. Invertebr. Pathol. 120, 43–49.
SAS Institute Inc. 2018. 9.4 In-Database Products, User’s Guide, fifth ed. SAS Publishers, Cary, NC, USA.
Sharma A, Jaronski S, Reddy GVP. 2019. Impact of the granular carriers to improve the efficacy of entomopathogenic fungus against wireworms in spring wheat. Journal of Pest Science. DOI: 10.1007/s10340-019-01161-1.
Sharma A, Sandhi RK, Briar SS, Miller JH, Reddy GVP. 2018. Assessing the performance of pea and lentil at different seeding densities as trap crops for the management of wireworms in spring wheat, Journal of Applied Entomology, 143,460–469.