Exploring the possibilities of entomopathogenic nematodes for wireworm management (Coleoptera: Elateridae)
Aim of the study
The different aims of the study were: (1) To test the efficacy of available EPN strains against wireworms in shade house and field, (2) To identify the native EPN strains in Golden Triangle Region of Montana, (3) To test the efficacy of native EPN strains against wireworms in shade house.
Materials and methods
Wireworm Collection: The larvae of different instars were collected from different locations (Conrad, Pendroy, and Kallispell fields). The wireworms were collected by using stocking traps. The stocking traps with soaked wheat seeds were placed in the different spots in soil and then covered with plastic sheets. After 15-20 days, the stocking traps were collected and brought back to the laboratory. These stocking traps were replaced every time we collected the old traps. The stocking traps with wireworms were placed in the Berlese Funnels for 12 hours and the wireworms were collected and categorized into small, medium and large based on their size. We found mainly three wireworm species viz. Limonius californicus, Hypnoides bicolor, and Aeolus mellillus. However, the wireworm specie, L. californicus is the dominant specie in this area, and subsequently used in further experiments. Wireworms were stored in 5 oz. plastic cups in sterilized sandy loam soil with wheat seeds as their food. These plastic cups were placed in an incubator at 8°C.
Objective 1
-
-
Efficacy of available EPN strains against wireworms in shade house and field Material and methods
Ten nematode strains were obtained from Dr. David Shapiro (USDA ARS, Georgia). The list is given in Table 1 as follows:
Table 1. List of EPN strains (Source: Dr. David Shapiro, USDA-ARS, Georgia)
Entomopathogenic nematode species
Strain
Steinernema carpocapsae
All strain
Cxrd strain
Steinernema feltiae
SN strain
Heterorhabditis bacteriophora
HP88 strain
VS strain
Steinernema riobrave
355 strain
7-12 strain
Heterorhabditis floridensis
K22 strain
Heterorhabditis georgiana
Kesha strain
Steinernema rarum
17 c + e strain
Four EPN strains (S. carpocapsae All and Cxrd strains and S. riobrave 355 and 7-12 strains) were found effective against L. californicus in the laboratory bioassay and shade house experiment in 2018. These four EPN strains were further tested against L. californicus in shade house experiment with modifications as well as field in 2019.
Nematodes rearing: Ten waxworm larvae were placed on filter paper in the petri dishes. 500- 1000 Infective Juveniles (IJ)/ml for different strains were inoculated in the petri dishes with 100- 200 IJ/waxworm larva. The petri dishes were left at room temperature for nematode infection. After 3-5 days, nematodes infected larvae were placed on the white traps for rearing. After 7 to 10 days, nematodes were collected from the white traps and stored in the tissue culture flasks.
Shade house screening: The experiment was conducted in a completely randomized block design with four EPN strains based on their efficacy in laboratory and shade house experiment in 2018. Plastic pots (16 cm diameter) were filled with approximately 2.4 kg of sterilized field-collected sandy loam soil (depth: 14 cm) with a surface area of 200 cm2. The soil used was sandy loam soil (78% sand, 12% silt and 10% clay, pH 7.7, and 1.4% organic matter). Ten wheat seeds were planted in each pot and allowed to grow for 10 days. Ten wireworms were added to each pot after 10 days. After a further 24 hours, any larvae that did not enter the soil were replaced. Two concentrations [80,000 IJs/pot (400 IJs/cm2) and 10,000 IJs/pot (50 IJs/cm2)] were used for each of the four strains. These two concentrations were prepared according to Navon and Ascher (2000) and standardized as 4000 IJs/ml and 500 IJs/ml of tap water. The IJs, in 20 ml of water were inoculated into the pots with a pipette. The pots with the control treatment received 20 ml of plain water. There were five replicates (pots) for each concentration. The pots were placed in a shade house and watered daily (Figure 1). After 4 weeks, the pots were destructively sampled and the number of dead wireworm larvae observed. The dead larvae were dissected to confirm nematode infection. If a wireworm was not found, it was recorded as dead. The average air temperature in the shade house was 30°C (26-32°C) with average soil temperature and soil moisture of 20°C (11- 35°C) and 21 ± 5%, respectively in pots. The whole experiment was conducted in two trials with an interval of 10 days in both trials. Plant damage, i.e. number of wheat seedlings damaged by wireworms, was observed in each pot at the end of the experiment and the average percentage of plant damage was recorded. The presence of wilted or dead central leaf and/or seedling death was the main criteria in observing plant damage.
Figure 1. Shade house screening of available EPNs against L. californicus
-
Efficacy of selected EPN strains against L. californicus in field EPN source and production of cadavers
-
Greater wax moth larvae, Galleria mellonella L. (Lepidoptera: Pyralidae) were obtained from the Bassett's Cricket Ranch (CA, USA). The larvae were stored in the containers provided by the supplier at 10°C until used for culturing. EPN infected G. mellonella cadavers were used as a source of nematodes in field rather than aqueous suspensions to mimic the natural conditions. Ten
G. mellonella larvae were exposed to approximately 200 freshly produced IJs of four selected EPN strains; S. carpocapsae (All and Cxrd strains) and S. riobrave (355 and 7-12 strains) in a 90 mm diameter Petri dish. These EPN strains were obtained from the USDA-ARS Entomopathogenic Nematode culture collection (Byron, GA). The petri dishes were held at room temperature (22°C) for 3-4 days. Overall, 80 cadavers were prepared for each EPN strain. The nematode infected cadavers were then transferred to individual White traps (Kaya and Stock 1997) to observe the initiation of IJs emergence for another 4-5 days at room temperature. The traps were checked daily for the initiation of IJs emergence from the cadavers. After 4-5 days, the cadavers that just started releasing IJs were used in field experiments to reduce the chances of variation due to initiation of emergence among replications.
Study sites
The field trials were conducted in a barley field (Pendroy: N48.04130°, W112.16945°) and a spring wheat field (Choteau: N47.9023°, W112.2330°) in Golden Triangle Region of Montana in 2019. Both the fields were selected on the basis of history of moderate to high wireworm pressure. The fields were tested to assure the presence/absence of naturally occurring EPN species and were found negative in respect to nematodes. According to NRCS (1999), the soils at Pendroy site had Rothiemay-Niart clay loams soil, with 0–4% slopes and Choteau site had Niart-Crago gravelly loams soil with 0–4% slopes (NRCS 1999).
Experimental design
The spring wheat (variety: Clear field) was seeded on May 9th 2019 and the barley field (Variety: Hockett) was seeded on May 10th 2019. The farmers at both the sites seeded the plots. The Choteau site (spring wheat) was managed as irrigated site and Pendroy site (barley) was managed under dryland farming practices. The row spacing was kept at 7.5 inch and 10 inch in spring wheat and barley fields, respectively. In spring wheat field, 15 gallons of ‘32’ nitrogen, N-P-K (20-10-5) at the rate of 140 gallons/acre, and 25 tons of manure at the rate of 120 pound/acre were applied before seeding. The herbicides Beyond and Wildcard were applied at the label rate for weed control. Imidacloprid was used as seed treatment in both fields. In addition, Roundup at the rate of 16-20 ounces and liquid nitrogen at the rate of 5 gallon were applied before seeding.
The experiment was completely randomized block design including 33.7 m long and 17.6 m wide field plot with five 33.7 m × 1.52 m sections with 9 subplots (1.52 m × 1.52 m) in each plot. Each sub plot had four to five plant rows. There was 2.5 m buffer zone between each subplot in one row of nine subplots, to avoid the inter-specific competition between the EPN strains. Similarly, there was 2.5 m buffer zone between four rows of 33.7 m × 1.52 m sections to avoid effects from migration of EPNs and plot order was randomized at each location.
Two doses (3 and 6 cadavers per subplot) were tested with five replications. There were 5 replications for control subplots without any cadavers. Overall, there were 45 subplots (4 strains × 2 doses × 5 replications = 40 subplots + 5 subplots (Control)). A hand shovel was used to dig the soil and the cadavers were placed 5-8 cm beneath the soil surface and at least 10 cm away from each other five days after seeding. The holes with cadavers were covered with soil thereafter. In spring wheat field, cadavers were released in early morning at 9:00 AM in cloudy conditions with an average soil temperature of 5.5±4°C, average air temperature of 16°C, and average soil moisture percentage of 23.7%. However, in barley field, EPN cadavers were released in the evening at 7:00 PM in cloudy conditions with an average soil temperature of -6±2°C, average air temperature of 23°C, and average soil moisture percentage of 59%.
Plant Count
To observe the wireworm damage to wheat plants, number of seedlings in each plot were randomly counted using 1 m line intercept method. Two rows were selected from each plot and both ends of each row (1 m length) were marked with iron nails for plant counting. The first count from both rows (n=2) was taken three weeks after plant germination. The second and final plant count was made just before harvesting. At harvest, the height of these same marked plants was recorded using a wooden meter scale (Washington, USA).
Number of Wireworms/Wireworm sampling
Soil bait traps as explained by Reddy et al. (2014) were used to determine the wireworm density in the experimental plots. The traps were placed in the soil and collected back from the field in plastic bags, labeled and brought back to the Western Triangle Agricultural Research Center (WTARC), Conrad laboratory for extraction. The larvae were sorted from the traps manually using Berlese funnels (Bioquip products, California, USA, built at WTARC). The collected wireworms were counted and identified using the Etzler (2013) key for wireworm identification. The traps were placed in both field twice a month starting 10 days after barley seeding in Choteau and 20 days after spring wheat seeding in Pendroy. The reason for delay in trap placement in Pendroy field was excessive rain in the field. The traps were replaced five times in both fields from June to August at two weeks intervals. Soil temperature and soil moisture were also recorded at the time of wireworm trap collection by using Soil Thermometer (Taylor, IL, USA) and soil moisture meter (Spectrum Technologies Inc., IL, USA), respectively. Air temperature was also checked every time the traps were replaced.
Emergence of IJs from cadavers
To determine IJ emergence rates from cadavers applied in the field, randomly selected 15 infected cadavers were removed from the treatment batches for all the four EPN strains and placed individually on separate white traps at room temperature. Emerging IJs were collected, washed with tap water for 2-3 times, counted by serial dilution method by following the procedure of Navon and Ascher (2000). The emerging IJs were collected and counted until emergence stopped (3 weeks).
IJs persistence
The IJs persistence or survival of EPN IJs was observed in the treatment plots in August 2019 (one month before harvesting). Five soil core samples (approx. 100 g each) were taken from each plot by a hand shovel and mixed together to make a composite sample. The hand shovel was washed with water and rinsed with 75% ethanol in between plots to avoid contamination. Overall, there were 45 composite samples from each plot. These composite samples were kept in plastic bags separately in a thermal cooler and brought back to WTARC laboratory. EPN IJs were recovered from the soil samples using the insect baiting technique (Bedding and Akhurst 1975). Approximately 300 g soil sample from each composite sample was transferred to a 500 ml plastic container with ten G. mellonella larvae in each cup. The containers were kept in the dark at room temperature (22 ± 2°C). After seven days of incubation, the dead larvae were removed and rinsed with tap water. The dead larvae that showed signs of infection with EPNs, i.e. placid soft odorless larvae with either pale yellowish to brown or black color were recorded as dead because of EPN infection. The dead larvae were also dissected to confirm the IJ presence. The dead G. mellonella larvae were averaged over the replication to observe the mean larval mortality.
Post-harvest data collection
After harvesting, wheat and barley grains from each plot were brought to the WTARC facility and cleaned using a seed cleaning machine (Almaco, Allan Machine Company, IA, USA). The plot and test weight were measured using a laboratory balance (Ohaus, Adventure™ Pro model AV8101). Wheat and barley samples were processed through a grain analyzer (Perten instruments IM9500; Hägersten, Sweden) to determine grain moisture and protein. About 300 g of sample for each plot was processed to obtain protein and moisture content. Plot weight and moisture were used to calculate yield.
Objective 2
-
To identify the native EPN strains in Golden Triangle Area of Montana
In 2018, soil samples were collected from 30 different fields from Pendroy, Choteau, Valier, Conrad, Kallispell, Knees, Brady, Collins, Dutton, Shelby, Sunburst, and Tiber areas were collected in Golden Triangle Area of Montana. These soil samples were observed for presence of naturally occurring EPNs in Montana. Different samples were found positive with nematodes.
However, the nematodes collected from some soil samples were not able to reproduce further indicating that these might be other bacterivore nematodes other than EPNS. Overall, three EPN species were found from all the soil samples collected. These isolates were sent to Monte L. Bean Museum, and Evolutionary Ecology Laboratories, Brigham Young University, Provo for molecular identification.
-
Molecular identification
For DNA extraction, pooled EPN IJs of each isolate were macerated with a plastic pestle in 1.5 ml centrifuge tube and genomic DNA was extracted using Qiagen DNeasy® Blood and Tissue kit (Waltham, MA) by following manufacturer’s protocol. The extracted DNA was concentrated to 20 µl using Eppendorf Vacufuge Plus Vacuum Concentrator (Hamburg, Germany). A part of rDNA comprising the internal transcribed spacer regions (ITS), ITS1 and ITS2 including 5.8S were sequenced using two sets of primers. Primer set ITS-F (5’-TTGAACCGGGTAAAAGTCG- 3 and ITS-R (5’-TTAGTTTCTTTTCCTCCGCT-3’) was used to sequence the entire ITS1, 5.8S and ITS2 regions while primer set Fnema18S (5’-TTGATTACGTCCCTGCCCTTT-3’) and rDNA1.58S (rev) (5'-ACGAGCCGAGTGATCCACCG-3') pair targeted the ITS1 region. Each PCR reaction was carried out in a total volume of 30 µl consisting of 9 µl of DNA template, 15 µl of JumpStart™ REDTaq® ReadyMix (Sigma-Aldrich, St. Louis, MO), 2.4 µl of each primer and1.2 µl of molecular grade water. The PCR conditions included initial denaturation at 940C for 5 minutes, 40 cycles of denaturation at 940C for 30 s, 40 cycles of annealing at 480C for 30 s, 40 cycles of extension at 0.50C/sec for 90 s and a final extension at 720C for 5 minutes. The PCR products were analyzed for expected DNA band weights on 1% agarose gel run at 150V for 20 minutes. PCR products were treated with ExoSAP-IT™ PCR Product Cleanup Reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol to digest excess primers and nucleotides. The products were sequenced bidirectionally with their PCR primers using Bigdye reaction chemistry on an ABI ABI3730xl. Primer sequences were removed from chromatograms, aligned and edited manually in Geneious Prime 2019.2.1 (http://www.geneious.com). Each species was identified via BLASTn (NCBI; http://www.ncbi.nlm.nih.gov) against the nucleotide collection (nr/nt) database using default search parameters.
Overall, 18 samples out of 150 samples were found to have nematodes. However, we were able to culture only three samples. This might be due to a number of factors like Galleria as inappropriate host for some particular species, unfavorable environmental conditions in the laboratory, low number of nematodes present in the soil samples to infect the host. Three nematode species were named as KP, MLS1, and MLS2 and identified using morphological and molecular techniques. Both species named KP and MLS1 were found to be Steinernema feltiae and MLS2 was identified as Heterorhabditis bacteriophora.
Objective 3: To test the efficacy of native EPNs against wireworms in laboratory and shade house
-
Efficacy of native EPNs against medium sized L. californicus larvae (Laboratory concentration response assay)
Heterorhabditis bacteriophora, S. feltiae 1 and S. feltiae 2 were tested against medium sized L. californicus larvae in a laboratory bioassay. 500 ml plastic cups were filled with 150 gm of soil (soil surface area: 114 cm2). The soil used was sandy loam soil (78% sand, 12% silt and 10% clay, pH 7.7, and 1.4% organic matter). Before use, the soil was autoclaved at 121°C for 1 hr and left at room temperature for one week for acclimatization. Five medium sized wireworm larvae were introduced into each cup with eight to ten germinated wheat seeds as food. The larvae that did not enter the soil within 12 hrs were replaced. Four concentrations [3500 IJs/cup (30 IJs/cm2), 7000 IJs/cup (60 IJs/cm2), 14,000 IJs/cup (120 IJs/cm2), and 28,000 IJs/cup (240 IJs/cm2)] were tested for all three test EPN species. The concentrations were prepared by counting the desired number of IJs into 100 μl to 1 ml of tap water (depending on the concentration) in a nematode counting slide (Chalex, LLC, Park City, UT, USA) under a compound microscope by following Navon and Ascher (2000) formula. Ultimately, four doses for three species were standardized and adjusted as 5600, 2800, 1400, and 700 IJs per ml of water and stored in the tissue culture flasks at 8°C in an incubator. The IJs were used within 15 days of culturing.
Before application, EPNs were transferred from 8°C to room temperature for 12 h for acclimatization. The viability of IJs was checked under the microscope before application. The IJs were pipetted onto the soil surface in 1 ml of IJs suspension, and the control cups received one ml of tap water without any IJs. The final soil moisture was adjusted to 18% (v/w) that may be prevalent in Montana soils during EPN application. There were five replications for each of the four concentrations for all EPN species. The bioassay was conducted twice, on various dates from June to August 2018. The cups were placed in trays with approximately 10 holes in the lids and placed in an incubator at 22°C and 75% RH in the dark. 22°C temperature in the bioassay is an average temperature during the wireworm activity in the field and can be targeted for EPN application. Larval mortality was observed weekly for four weeks.
-
Effect of soil texture on the efficacy of native EPNs against L. californicus in laboratory Steinernema feltiae 1 and S. feltiae 2 isolates were tested in this laboratory bioassay. Four different types of soils as described in Table 1 were used in this study. Different soils were sterilized in an autoclave at 121°C for 1 hr to kill natural nematode populations and other microorganisms. The soils were left at room temperature for at least two weeks for acclimatization. 500 ml plastic cups were filled with 150 gm of soil (soil surface area: 140 cm2) for different soils. Five medium sized wireworm larvae were introduced into each cup with 8-10 germinated wheat seeds as food. The larvae that did not enter soil within 12 hrs were replaced. After 24 hrs, 7000 IJs/cup (50 IJs/cm2) were inoculated in one ml of water into each cup. This dose was prepared by following the same procedure as mentioned in section 2.3. Control cups received only one ml water without IJs. The final moisture content was standardized at 18% (v/w) for all the soil types. The reason for standardizing the moisture was to compare the EPN efficacy at same moisture level. After inoculation, the cups were placed in an incubator at 22°C and 75% RH in dark conditions. The moisture was provided every two to three days to maintain the moisture in the cups. The wireworm mortality was assessed at weekly intervals for four weeks. There were five replications for each treatment. The experiment was conducted twice with an interval of two weeks between trials and different nematode cultures were used for both trials.
-
Efficacy of selected EPN strains against L. californicus larvae in shade house trial
The experiment was conducted in a completely randomized block design with S. feltiae 1 and S. feltiae 2 as treatments. Plastic pots (14 cm diameter) were filled with approximately 1.7 kg of sterilized field-collected sandy loam soil (depth 9 cm) with a surface area of 150 cm2. The soil was 78% sand, 12% silt, and 10% clay, with pH 7.7 and 1.4% organic matter. Ten wheat seeds were planted in each pot and allowed to grow for 10 days. Five wireworms were added to each pot after 10 days. After further 24 hours, any larvae that did not enter the soil were replaced. Two concentrations [60,000 IJs/pot (400 IJs/cm2) and 7,500 IJs/pot (50 IJs/cm2)] were used for each of the two S. feltiae isolates. These two concentrations were standardized as 6000 IJs/ml and 750 IJs/ml of tap water. The IJs, in 10 ml of water were inoculated into the pots with a pipette. The pots with the control treatment received 10 ml of plain water. There were five replicates (pots) for each concentration. The pots were placed in a shade house and watered daily. After four weeks, the pots were destructively sampled and the number of dead wireworm larvae observed. The dead larvae were dissected to confirm nematode infection. If a wireworm was not found, it was recorded as dead. Soil temperature and moisture were observed three times each week throughout the experiment using a soil moisture meter (Spectrum Technologies Inc., Illinois, USA) and soil thermometer (Taylor, Illinois, USA). The average air temperature in the shade house was 31°C (26-35°C) with average soil temperature and soil moisture in the pots was recorded as 22°C (11- 35°C) and 21±5%, respectively. The whole experiment was conducted in two trials from May to August 2019 with an interval of 10 days in both trials. Plant damage, i.e. number of wheat seedlings damaged by wireworms, was observed in each pot at the end of the experiment and the average percentage of plant damage was recorded. The presence of wilted or dead central leaf and/or seedling death was the main criteria in observing plant damage.
Statistical Analysis: GLM with binomial/quasibinomial distribution was used for the laboratory and shade house experiments in case of both available and Montana native EPNs. The data for EPN efficacy against wireworms were analyzed separately for both field because crops were different in both fields. The data regarding IJs emergence in the laboratory, number of wireworms collected, yield, plant count, plant height, test weight, moisture and protein content were subjected to Analysis of variance. Wireworm number data in Pendroy site was normalized using log transformation. The data with number of Galleria mellonella larvae infected with EPNs in the soil samples taken from the field were analyzed using GLM with quasibinomial distribution to avoid the overdispersion problem. Tukey-Kramer test was used to get the significant differences between the treatments. Data were analyzed using the software statistical package R 2.15.1 (R Development Core Team, 2017).
Results and discussion
-
Efficacy of available EPN strains against wireworms in shade house in 2019
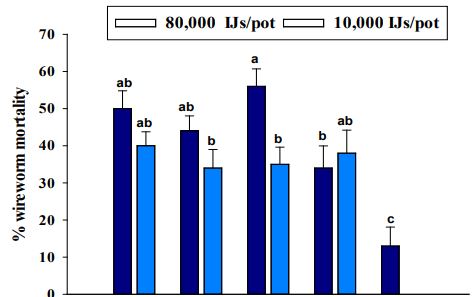
The wireworm mortality proportions differed significantly between the two trials (χ2 = 26.37, df = 1, P <0.0001), but interaction between trail and strain was not significant (χ2 = 2.795, df = 4, P=0.59) and therefore the data for two trials were pooled for further analysis. EPN strains had a significant impact on wireworm mortality (χ2 = 91.89, df = 4, P <0.0001). Wireworm mortality also differed significantly among concentrations (χ2 =8.43, df = 1, P = 0.004). However, there was no significant interaction between EPN strain and concentration (χ2 = 9.36, df = 4, P = 0.052). There were no significant differences among EPN strains in terms of wireworm mortality (Figure 2). The wireworm mortality due to EPN strains ranged from 34 to 56% when applied at 80,000 IJs/pot with 56% and 50% mortality caused by S. riobrave 355 and S. carpocapsae All strains, respectively. The mortality percentage was 35-40% when EPNs were applied at the rate of 15,000 IJs/pot. However, the wireworm mortality was significantly lower in control (13%).
The two trials in 2019 differed significantly in regards to the rate of plant damage (χ2 = 22.99, df
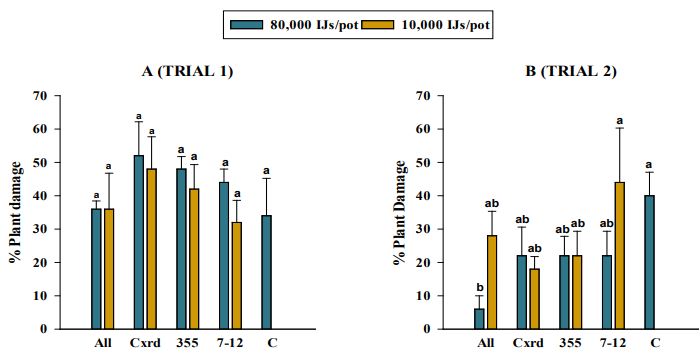
= 1, P <0.0001), and were therefore analyzed separately. In trial one, plant damage was not significantly affected by EPN strain (χ2 = 7.42, df = 4, P = 0.12) or concentration (χ2 = 1.02, df = 1, P = 0.312). No significant interaction was found between EPN strain and concentration (χ2 = 1.04, df = 4, P = 0.904). None of the four EPN strains tested differed significantly from that of the control, all being in the range of 34-52%, the highest level (52%) being caused when S. carpocapsae Cxrd was applied (Figure 3A). However, in trial two, EPN strain and concentration both had significant effects on plant damage (EPN strain: χ2 = 19.29, df = 4, P = 0.0007; Concentration: χ2 = 4.29, df = 1, P = 0.038). In addition, a significant interaction was observed between EPN strain and concentration (χ2 = 10.69, df = 4, P = 0.030). Steinernema riobrave 7-12 strain was associated with the highest plant damage at 44% followed by 40% plant damage in the control treatments (Figure 3B). For S. carpocapsae All, S. carpocapsae Cxrd, and S. riobrave 355 plant damage did not exceed 28%.
-
Efficacy of selected EPN strains against L. californicus in field
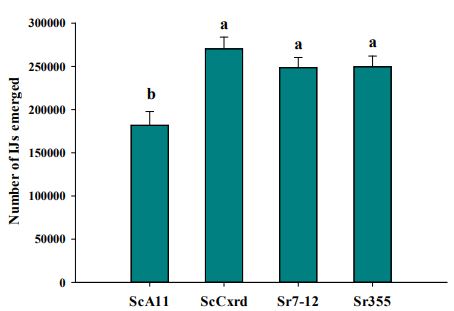
In June, the Pendroy (barley) and Choteau (spring wheat) sites had soil temperature varied from 6-14±5°C with 15-20°C air temperature. In July, both sites were observed with 15-22±5°C soil temperature and 22-27°C air temperature. In beginning of August, the soil temperature was higher in Pendroy site (20±5°C) with 16±2°C soil temperature at Choteau site. However, the soil moisture varied between two sites. At Pendroy site, the soil moisture content was almost twice as compared to Choteau site. The soil moisture content at Pendroy site was 55.8-56.2±3%, 43-48±5%, and 55.38±5% in June, July, and first week of August, respectively. However, the soil moisture content at Choteau site was 22-29±5%, 25.88-32.24±7%, and 31.02±8% in June, July, and August, respectively. The IJs emerged from one cadaver in the laboratory varied significantly among four EPN strains (F=8.55, df=3, p<0.0001). EPN strains S. carpocapsae Cxrd, S. riobrave 7-12 and S. riobrave 355 produced significantly higher number of IJS (248,500 to 270,270) as compared to 181,860 IJs produced by S. carpocapsae All strain (Figure 4).
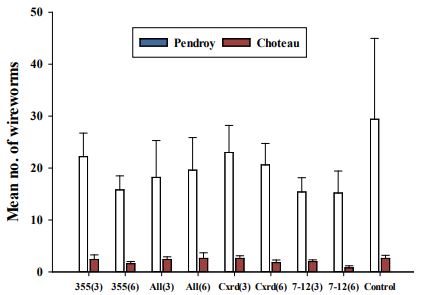
The data were analyzed separately for both sites, as the crops were different at both sites. At Pendroy site, all the three wireworm species were found with L. californicus as the dominant species with greater numbers (~75%) followed by A. mellilus (~15%) and H. bicolor (~10%). However, at Choteau field site with barley, only H. bicolor was observed. Wireworm larvae found at both the field sites were of almost all the instar stages. The data regarding total number of wireworms collected throughout the season were statistically analyzed. However, the data for number of wireworms collected at different time intervals are provided in Table 2. The wireworm pressure was high in Pendroy site as compared to Choteau site (Figure 5 and Table 2). Overall, in Pendroy, wireworm number trends remained almost same from June to July but more wireworms were collected in the beginning of August (Table 2). However, the number of wireworms collected remained same throughout the collection time in Choteau.
At Pendroy site, total mean number of total wireworms (log transformed) collected in the season did not differ significantly (Figure 5) among EPN strains (F=0.62, df=4, p=0.65) and dose (F=0.11, df=1, p=0.74). The interaction between EPN strains and dose was also non-significant (F=0.12, df=4, p=0.97). Similar trend was seen at Choteau site where wireworm number did not vary significantly due to EPN strains (F=1.20, df=4, p=0.33) and dose (F=1.78, df=1, p=0.19). The interaction between EPN strain and dose was also not significant for wireworm numbers (F=0.46, df=4, p=0.76). There was a major problem of weeds and volunteer plants at both the sites and plant count done at three weeks after planting could not be considered accurate further. Therefore, data for plant count before harvesting is being analyzed further. At Choteau site, no parameters (yield, test weight, plant count, plant height, moisture, and protein) varied significantly due to different treatments and doses (Table 3). Similarly, none of the post-harvest and other parameters (yield, test weight, plant count, plant height, moisture and protein) differed significantly among EPN strains and doses (Table 4).
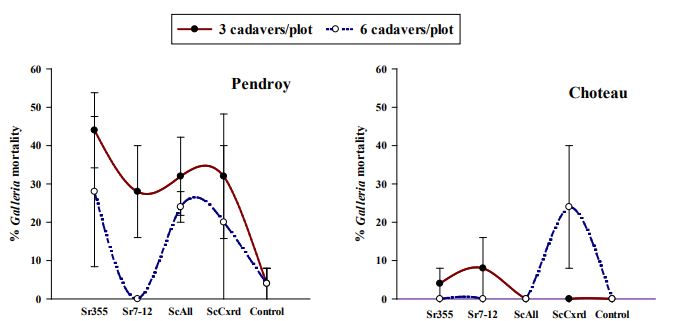
The data regarding number of Galleria mellonella larvae infected in soil samples taken were analyzed separately for both fields. At Pendroy site, EPN dose had a moderate level of effect on the Galleria mortality (χ2=3.52, df=1, p=0.06). However, EPN strain alone and the interaction of EPN strain and dose did not have a significant effect on the Galleria mortality (EPN- χ2=6.94, df=4, p=0.14; EPN:Dose- χ2=3.34, df=3, p=0.34). However, at Choteau site, EPN strains had significant effect on the Galleria mortality (χ2=17.30, df=4, p=0.002). The interaction between EPN strain and dose was also significant (χ2=16.44, df=3, p<0.0001). However, dose did not have significant effect on the Galleria mortality (χ2=1.50, df=1, p=0.22). Overall, the Galleria percentage mortality was very low at Choteau site as only 25% average mortality was observed in samples collected from plots with S. carpocapsae infected cadavers (Figure 6). However, at Pendroy site, the Galleria mortality was observed to be higher than Choteau site. There were no significant differences among EPN strains in terms of percentage larval mortality at Pendroy site. The percentage Galleria mortality ranged from 30-45% at Pendroy.
Overall, EPNs applied in the form of infected cadavers were observed not to prevent wireworm damage in crops as well as protecting yields at both sites.
Objective 3. To test the efficacy of native EPNs against wireworms in laboratory and shade house
-
Efficacy of native EPNs against medium sized L. californicus larvae (Laboratory concentration response assay)
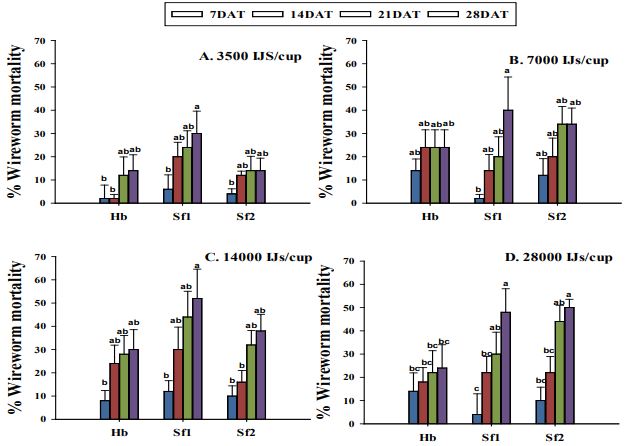
No three-way interaction was found between EPN strain, dose, and time to mortality (χ2=14.81, df=27, p-value=0.97), and therefore this factor was also removed from the model. There were significant differences among four EPN strains (χ2=445.38, df= 3, p-value<0.0001), dose (χ2=59.13, df= 3, p-value<0.0001), and time (χ2=171.22, df= 3, p-value<0.0001) with respect to wireworm larval mortality. The interaction between EPN strain and dose (χ2=24.26, df= 9, p- value=0.004) and EPN strain and time to mortality (χ2=16.96, df= 9, p-value=0.04) had significant effect on the wireworm larval mortality proportions. However, no significant interaction was detected between dose and time (χ2=2.13, df= 9, p-value=0.98) on the wireworm larval mortality. No wireworm mortality was observed in the control treatment. On average, wireworm larval mortality increased with higher nematode concentrations, from 3500 to 28,000 IJs/cup. After one week, the larval mortality did not even exceed 15% in all the three tested EPNs at all the concentrations applied (Figure 7). This mortality trend remained almost the same after two weeks with mortality ranging from 12 to 30% with 30% mortality caused by S. feltiae 1. However, the larval mortality increased after three weeks of treatment. When EPNs were applied at the rate of 28,000 IJs/cup, Steinernema feltiae (1 and 2) caused significantly higher (48-50% mortality) as compared to only 24% mortality caused by H. bacteriophora after four weeks of treatment. Steinernema feltiae 1 and 2 isolates and H. bacteriophora did not differ significantly in terms of wireworm mortality when applied at 14,000 IJs/cup with 52% mortality caused by S. feltiae 1.
-
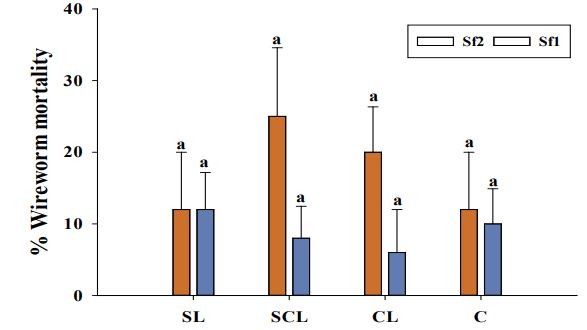
Effect of soil texture on the efficacy of native EPNs against L. californicus in laboratory No significant differences observed between two trials in terms of L. californicus larval mortality [(trial: χ2=0.99, df= 1, P=0.318); (trial:strain: χ2=0.07, df= 2, P=0.97)], therefore the data were pooled together for further analysis. The interaction between EPN strain and time on wireworm mortality was not significant (χ2=1.55, df= 6, P=0.96), therefore data for cumulative wireworm mortality after 4 weeks of treatment is being analyzed further. Two EPN strains had significant effect on L. californicus mortality (χ2=74.29, df=2, P<0.0001). However, wireworm mortality caused by different EPN strains did not differ significantly among four soil types (χ2=0.36, df= 3, P=0.95). In addition, no significant interaction was observed between EPN strain and four soil types (χ2=2.54, df= 6, P=0.86). No wireworm mortality was observed in the control treatment. Similarly, the wireworm mortality caused by two isolates of S. feltiae did not differ significantly from control (P>0.05) in different soil types (Figure 8). Overall, the wireworm mortality caused by S. feltiae did not exceed 25%.
-
Efficacy of selected Montana native EPN strains against L. californicus larvae in shade house trial
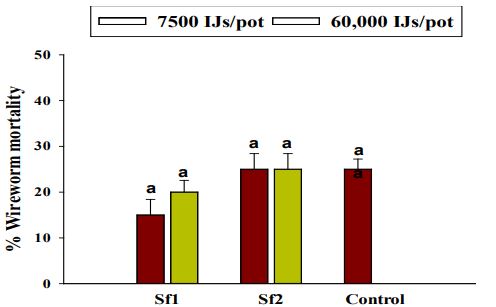
There were no significant differences observed between two trials repeated in time in respect to L. californicus larval mortality (χ2=0.621, df= 1, P=0.430), therefore the data were pooled together for further analysis. EPN strains had significant effect on the wireworm mortality proportions (χ2=6.64, df= 2, P=0.04). However, two doses did not differ significantly in regards to wireworm mortality proportions (χ2=1.20, df= 1, P=0.27). Additionally, no significant interaction was detected between EPN strains and dose (χ2=0.65, df= 2, P=0.72) on wireworm mortality. Steinernema feltiae 1 and S. feltiae 2 did not differ significantly from control in regards to L. californicus mortality (Figure 9). Overall, L. californicus mortality ranged from 15 to 25% when EPNs were applied at the rate of 60,000 IJs/pot and 7500 IJs/pot.
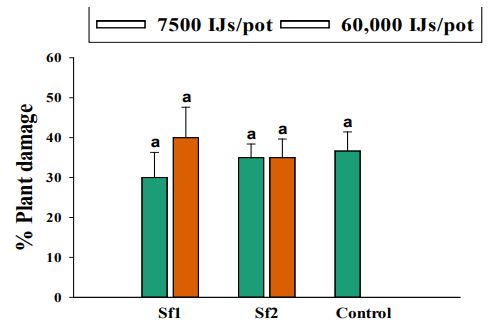
Two trials repeated in time did not differ significantly in respect to plant damage caused by L. californicus (χ2=3.67, df= 1, P=0.06). Similarly, the interaction between EPN strains and trials was not significant (χ2=3.77, df= 2, P=0.15), therefore the data were pooled together for further analysis. EPN strains did not have significant effect on plant damage (χ2=0.14, df= 2, P=0.93). L. californicus larval mortality did not differ between two doses applied (χ2=0.63, df= 1, P=0.43). No significant interaction was detected between EPN strains and the doses applied (χ2=1.27, df= 2, P=0.53). None of the two isolates of S. feltiae tested differed significantly from control treatment in respect to plant damage (Figure 10). The plant damage caused by L. californicus in the presence of S. feltiae ranged from 30-40%. Limonius californicus larvae were able to cause 36% plant damage in control treatment as well.
Figure 2. Average percentage mortality of larval Limonius californicus after exposure to entomopathogenic nematodes at 80,000 Infective Juveniles (IJs)/pot and 10,000 IJs/pot in shade house in 2019. All = Steinernema carpocapsae All; Cxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7-12 = Steinernema riobrave 7-12; Con = Control. Different letters above bars indicate statistical significance (P≤0.05, Tukey-Kramer test).
Figure 3. Average percentage plant damage by larval Limonius californicus after exposure to entomopathogenic nematodes at 80,000 Infective Juveniles (IJs)/pot and 10,000 IJs/pot in trial 1 (A) and trial 2 (B) in shade house in 2019. All = Steinernema carpocapsae All; Cxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7-12 = Steinernema riobrave 7-12; Con = Control. Different letters indicate statistical significance (P≤0.05, Tukey-Kramer test).
Figure 4. Mean number of IJs emerged from a cadaver infected with different EPN strains where where ScAll = Steinernema carpocapsae All; ScCxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7-12 = Steinernema riobrave 7-12.
Figure 5. Total mean numbers of wireworms collected in bait traps after 75 days in 2019 [n=5]. No significant difference was found between treatments (α = 0.05, Tukey-Kramer test), where ScAll = Steinernema carpocapsae All; ScCxrd = Steinernema carpocapsae Cxrd; Sr355= Steinernema riobrave 355; Sr7-12 = Steinernema riobrave 7-12.
Table 2. Number of wireworms collected from treatment plots in 2019.
|
Pendroy (Barley)* |
||||||
|
Treatment |
12 June 2019 |
27 June 2019 |
10 July 2019 |
25 July 2019 |
9 August 2019 |
Total |
|
Sr7-12(3) |
12 |
9 |
10 |
16 |
30 |
77 |
|
Sr7-12(6) |
9 |
15 |
9 |
21 |
22 |
76 |
|
Sr355(3) |
3 |
26 |
16 |
30 |
36 |
111 |
|
Sr355(6) |
13 |
14 |
5 |
12 |
35 |
79 |
|
ScAll(3) |
13 |
17 |
13 |
24 |
24 |
91 |
|
ScAll(6) |
7 |
23 |
9 |
21 |
38 |
98 |
|
ScCxrd(3) |
2 |
12 |
9 |
35 |
57 |
115 |
|
ScCxrd(6) |
9 |
21 |
22 |
25 |
26 |
103 |
|
Control |
18 |
33 |
14 |
26 |
56 |
147 |
|
Choteau (Spring wheat) |
||||||
|
Treatment |
3 June 2019 |
18 June 2019 |
3 July 2019 |
17 July 2019 |
31 July 2019 |
Total |
|
Sr7-12(3) |
4 |
0 |
2 |
0 |
4 |
10 |
|
Sr7-12(6) |
0 |
0 |
1 |
2 |
1 |
4 |
|
Sr355(3) |
7 |
0 |
1 |
2 |
2 |
12 |
|
Sr355(6) |
0 |
2 |
3 |
1 |
2 |
8 |
|
ScAll(3) |
2 |
4 |
2 |
3 |
1 |
12 |
|
ScAll(6) |
3 |
4 |
0 |
2 |
4 |
13 |
|
ScCxrd(3) |
1 |
6 |
3 |
0 |
3 |
13 |
|
ScCxrd(6) |
2 |
4 |
1 |
1 |
1 |
9 |
|
Control |
2 |
3 |
3 |
3 |
2 |
13 |
*At Pendroy site, wireworm sampling was late for nine days as compared to Choteau site because of heavy rain at Pendroy during that nine days period.
Table 3. Plant count, plant height, moisture, protein, seed test weight, and yield in the EPN treated plots (Mean ± SE) in Choteau (Spring wheat) in 2019.
|
Treatment |
Plant Count |
Plant height (cm) |
Moisture (%) |
Protein (%) |
Test weight (kg/ha) |
Yield (kg/ha) |
|
Sr7-12(3) |
33.8±2.3a |
81.7±1.52 a |
11.89±0.30 a |
12.32±1.92 a |
77.89±1.17 a |
2050.15±330.91 a |
|
Sr7-12(6) |
37±2.07 a |
69.6±3.31 a |
11.79±0.06 a |
11.06±0.44 a |
81.19±1.83 a |
1525.90±160.81 a |
|
Sr355(3) |
34.5±2.80 a |
69.6±5.01 a |
11.42±0.16 a |
13.89±0.85 a |
76.49±1.69 a |
1495.58±265.91 a |
|
Sr355(6) |
36±3.5 a |
76.1±5.28 a |
11.82±0.23 a |
11.69±1.30 a |
79.05±0.98 a |
1598.19±371.44 a |
|
ScAll(3) |
34.6±2.5 a 8 |
74.1±3.59 a |
11.78±0.20 a |
11.90±1.27 a |
80.42±1.14 a |
1818.65±353.65 a |
|
ScAll(6) |
30.9±1.6 a |
70.3±3.52 a |
11.69±0.32 a |
13.28±1.61 a |
78.61±0.64 a |
1845.98±249.22 a |
|
ScCxrd(3) |
31.8±1.54 a |
68.0±3.84 a |
12.16±0.16 a |
10.70±0.67 a |
78.68±2.65 a |
1540.48±247.68 a |
|
ScCxrd(6) |
39.3±2.12 a |
79.7±2.66 a |
11.54±0.30 a |
14.73±1.61 a |
76.24±0.99 a |
1969.97±237.57 a |
|
Control |
38.7±1.68 a |
74.4±3.65 a |
11.80±0.30 a |
12.05±1.44 a |
77.07±1.37 a |
1689.78±106.56 a |
|
|
||||||
|
Treatment |
F4=1.74, p=0.16 |
F4=0.26, p=0.90 |
F4=0.18, p=0.95 |
F4=0.26, p=0.90 |
F4=1.22, p=0.32 |
F4=0.37, p=0.83 |
|
Dose |
F1=1.41, p=0.24 |
F1=0.04, p=0.85 |
F1=0.26, p=0.61 |
F1=0.18, p=0.68 |
F1=0.13, p=0.72 |
F1=0.002, p=0.97 |
|
Treatment:Dose |
F4=1.65, p=0.18 |
F4=3.03, p=0.03* |
F4=0.95, p=0.45 |
F4=1.49, p=0.22 |
F4=1.51, p=0.22 |
F4=0.88, p=0.49 |
Where ScAll = Steinernema carpocapsae All; ScCxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7- 12 = Steinernema riobrave 7-12. “3” and “6” represents number of cadavers. None of the treatment had significant effect on different parameters (α=0.05, Tukey-Kramer test).
Table 4. Plant count, plant height, moisture, protein, seed test weight, and yield in the EPN treated plots (Mean ± SE) in Pendroy (Barley) in 2019.
|
Treatment |
Plant Count |
Plant height (cm) |
Moisture (%) |
Protein (%) |
Test weight (kg/ha) |
Yield (kg/ha) |
|
Sr7-12(3) |
8.6±1.70 a |
79.3±6.82 a |
9.70±0.19 a |
12.3±0.72 a |
60.28±1.55 a |
1623.96±261.86 a |
|
Sr7-12(6) |
8.0±1.80 a |
79.4 ±4.92 a |
9.67±0.19 a |
12.59±0.89 a |
61.27±1.19 a |
2169.25±315.46 a |
|
Sr355(3) |
8.2±1.50 a |
72.6±5.81 a |
9.99±0.34 a |
12.71±0.59 a |
59.22±1.17 a |
1737.86±304.71 a |
|
Sr355(6) |
8.3±1.51 a |
71.8±2.91 a |
10.18±0.34 a |
12.54±0.80 a |
59.26±1.23 a |
1617.07±343.27 a |
|
ScAll(3) |
11.2±1.33 a |
84.3±5.01 a |
9.81±0.27 a |
12.64±0.53 a |
59.41±2.34 a |
2135.89±387.31 a |
|
ScAll(6) |
8.5±0.97 a |
80.0±5.77 a |
9.79±0.27 a |
11.94±0.48 a |
57.40±2.39 a |
1812.46±351.64 a |
|
ScCxrd(3) |
11.6±1.34 a |
91.5±4.23 a |
9.61±0.26 a |
11.84±0.54 a |
61.49±2.08 a |
1879.59±383.32 a |
|
ScCxrd(6) |
9±2.21 a |
76.8±8.48 a |
10.30±0.44 a |
13.39±1.12 a |
58.58±2.09 a |
1531.80±495.99 a |
|
Control |
9±1.88 a |
71.9±6.60 a |
10.13±0.12 a |
12.88±0.56 a |
58.83±1.72 a |
1860.02±261.80 a |
|
|
||||||
|
Treatment |
F4=0.62, p=0.65 |
F4=1.83, p=0.14 |
F4=1.04, p=0.40 |
F4=0.05, p=0.99 |
F4=0.56, p=0.70 |
F4=0.28, p=0.89 |
|
Dose |
F1=1.24, p=0.27 |
F1=1.11, p=0.29 |
F1=0.87, p=0.36 |
F1=0, p=0.99 |
F1=0.47, p=0.50 |
F1=0.15, p=0.70 |
|
Treatment:Dose |
F4=0.35, p=0.84 |
F4=0.57, p=0.69 |
F4=0.63, p=0.65 |
F4=0.67, p=0.61 |
F4=0.40, p=0.81 |
F4=0.59, p=0.67 |
Where ScAll = Steinernema carpocapsae All; ScCxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7- 12 = Steinernema riobrave 7-12. “3” and “6” represents number of cadavers. None of the treatment had significant effect on different parameters (α=0.05, Tukey-Kramer test).
Figure 6. Average percentage of Galleria mellonella infected with EPNs in collected soil samples. ScAll = Steinernema carpocapsae All; ScCxrd = Steinernema carpocapsae Cxrd; Sr355 = Steinernema riobrave 355; Sr7-12 = Steinernema riobrave 7-12. No significant differences were observed among the treatments (α=0.05, Tukey-Kramer test with GLM)
Figure 7. Average percentage mortality of larval Limonius californicus after exposure to entomopathogenic nematodes at 3500 (A), 7000 (B), 14000 (C), and 28000 (D) infective Juveniles/cup. Sf1 = Steinernema feltiae 1; Sf2 = Steinernema feltiae 2; Hb = Heterorhabditis bacteriophora. Different letters above bars indicate statistical significance (P≤0.05, Tukey-Kramer test); DAT=Days after Treatment; No larval mortality was observed in the control.
Figure 8. Average percentage mortality of larval Limonius californicus after exposure to entomopathogenic nematodes at 7000 infective Juveniles (IJs)/cup in shade house in 2019. Sf1 = Steinernema feltiae 1; Sf2 = Steinernema feltiae 2. Different letters above bars indicate statistical significance among EPN strains (P ≤0.05, Tukey-Kramer test); SL = Sandy loam; SCL = Sandy clay loam; CL = Clay loam; C = Clay. No larval mortality was observed in control treatments.
Figure 9. Average percentage mortality of larval Limonius californicus after exposure to entomopathogenic nematodes at 60,000 infective Juveniles (IJs)/pot (A) and 7500 IJs/pot (B) in shade house in 2019. Sf1 = Steinernema feltiae 1; Sf2 = Steinernema feltiae 2; Control= Control treatment. Different letters above bars indicate statistical significance among EPN strains (P ≤0.05, Tukey- Kramer test).
Figure 10. Average percentage plant damage by larval Limonius californicus after exposure to entomopathogenic nematodes at 60,000 Infective Juveniles (IJs)/pot and 7500 IJs/pot in shade house in 2019. Sf1 = Steinernema feltiae 1; Sf2 = Steinernema feltiae 2; Control= Control treatment. Different letters indicate statistical significance (P≤0.05, Tukey-Kramer test).
Acknowledgements
This work was supported by Montana Wheat and Barley Committee. We would like to thank all the farmers (Garrett Grub, John Stultz and Mike Lyne) for letting us use their fields for studies. We are thankful to Anamika Sharma, Ramadevi Gadi, Jonathan Blanchard, Shad Chrisman, and Julie Prewett for their help in different aspects of this study.
References
Bedding, R. A. and R. J. Akhurst. (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 21, 109–116.
Etzler, F.E., 2013. Identification of economic wireworms using traditional and molecular methods M.Sc thesis, Montana State University-Bozeman, College of Agriculture.
Kaya, H. K. and Stock, S. P. (1997) Techniques in insect Nematology. In: Lacey LA (ed) Manual of Techniques in Insect Pathology, pp 281±324. Academic Press, New York.
Navon, A., Ascher, K.R.S., (eds.) 2000. Bioassays of entomopathogenic microbes and nematodes. CABI international, Wallingford, Oxfordshire, UK, 324 pp.
NRCS (1999). Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Retrieved from https://websoilsurvey.sc.egov.usda.gov/.
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available: http://www.R-project.org. Cited 19 December 2017.